To the Editor: We would like to thank our colleagues for their thoughtful comments on our recent article and furthering the discussion on this critically important issue.
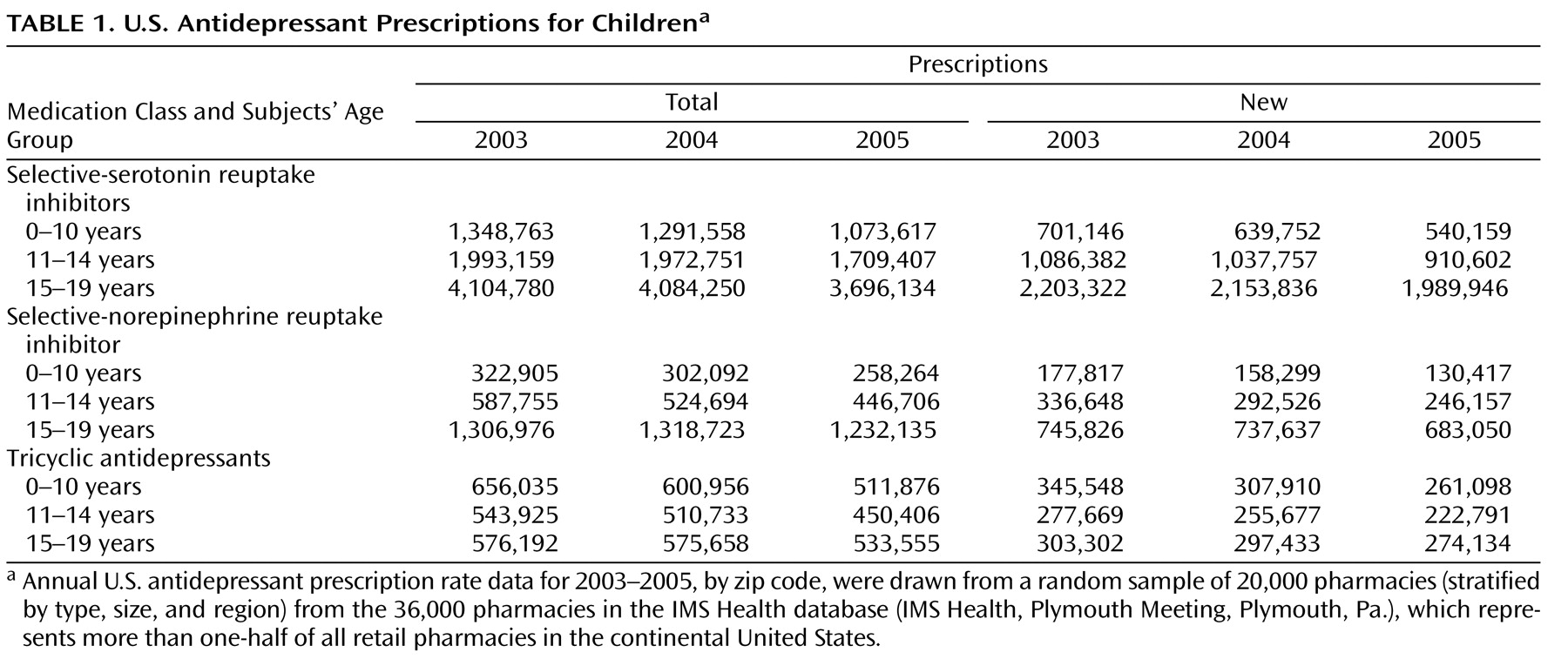
Dr. Jureidini suggests that we incorrectly concluded that decreases in prescriptions were associated with increases in suicide rates in children and adolescents. He notes that for the U.S. data, there was no significant drop in prescriptions in 2004, the last year for which suicide data in the United States were available at the time our study was published. However, our data reveal substantial decreases that already existed in 2004, particularly for new antidepressant prescriptions. As seen in
Table 1, among U.S. children and adolescents ages 0 to 19, total SSRI prescriptions decreased by 1.3%, but new prescriptions decreased by 4.0%. Similarly, total serotonin-norepinephrine reuptake inhibitor (SNRI) prescriptions decreased by 3.3%, and new SNRI prescriptions decreased by 5.7%. Total tricyclic antidepressant prescriptions decreased by 5.0%, and new total tricyclic antidepressant prescriptions decreased by 7.1%. Decreases in new antidepressant prescription rates for the younger children (under age 15) were even larger (6.2% for SSRIs, 12.4% for SNRIs, and 9.6% for total tricyclic antidepressants). These data are further detailed in an online data supplement that has been appended to the original article in the September issue (http://ajp.psychiatryonline.org/cgi/content/full/164/9/1356/DC1).
Decreases in antidepressant prescriptions following the FDA’s strong warning in March 2004 were reported throughout the remainder of 2004
(1,
2) using two different prescription databases that provided monthly prescription-rate data. In the first prescription database
(2), antidepressant prescription rates peaked in March 2004 and then dropped precipitously by 12.6%, in just one month after FDA’s Public Health Advisory on March 22nd, and continued to decrease gradually throughout the remainder of the year. In the second prescription database
(1), 20% decreases in antidepressant prescriptions from March 2004 to December 2004 were observed for children under age 18. In light of these findings, our overall comparisons between 2003 and 2004 underestimated the true effect of the FDA warning because they represent a combination of early increases in antidepressant prescriptions in the first 3 months of 2004 and decreases in the following 9 months of 2004. Furthermore, our analysis of the data from the Netherlands, where age-stratified data were available from 1998 to 2005 for both antidepressant prescription rates and suicide rates, identified a statistically significant inverse association between antidepressant prescription rates and suicide rates.
Dr. Jureidini also suggests that we neglected evidence that supports an iatrogenic or neutral effect of antidepressants on suicide. Our approach examined the potential net effect of antidepressants, the same standard that guides a risk-benefit analysis by physicians prescribing medication. Medications for cancer, diabetes, and heart disease may carry a risk of serious side effects and even death, but they may also save a lot more lives than they cost and are thus widely used. Moreover, we make no claim that antidepressant use is the only factor affecting youth suicide. Drug and alcohol use are well known risk factors, and their changing prevalence could partially explain some of the dramatic reductions in youth suicide rates that have occurred over the last decade; the limited changes in drug and alcohol use in recent years do not provide an explanation of the elevated youth suicide rates in 2004
(3) . In addition, our own data as well as that of others have indicated that there is still some elevated risk for suicide attempts during the first month after SSRI treatment is started
(4), and we agree with the FDA that this period is important for clinical monitoring.
Drs. Olfson and Shaffer consider it risky to draw conclusions from yearly changes in the suicide and antidepressant use data of two countries. We found the 14% increase in youth suicides from 2003 to 2004, along with sizable decreases in all antidepressant prescriptions, to be too large to ignore without commenting on the potential for early evidence of the effects of the FDA and European warnings. Drs. Olfson and Shaffer suggest that the FDA warnings could be associated with increases in non-SSRI antidepressant prescriptions. However, as described in our article as well as above, our data through 2004 do not support this contention. Indeed, the decreases that we observed for SSRIs are equal to or greater for SNRIs and total tricyclic antidepressants and even larger for new prescriptions than for total prescriptions. Decreases in SNRI and total tricyclic antidepressant prescription rates continue to parallel decreases found for SSRIs in 2005. Furthermore, in their study of one-half million adult depressive episodes, Valuck et al.
(5) showed that primary care physicians were less likely to make diagnoses of depression when the warnings began, and those individuals who were diagnosed were less likely to receive antidepressant prescriptions. These changes were not associated with compensatory increases in psychotherapy or prescription of atypical antipsychotics; anxiolytics did increase slightly, but not sufficient to compensate for the decreases in antidepressants.
Drs. Olfson and Shaffer also point out that one promising epidemiologic approach involves examining trends between psychotropic medication use and suicide over time across a large number of small geographic units. We agree that this is a very useful approach to the analysis of low-base rate events, and it is precisely the type of county-level analysis that we first used to examine the association between antidepressants and suicide
(6,
7), and it is also the type of statistical methodology that we introduced in the 2002 Institute of Medicine report “Reducing Suicide: A National Imperative”
(8) . Finally, Drs. Olfson and Shaffer refer to recently released preliminary suicide-rate data for 2005, indicating that suicide rates have not increased further from 2004 to 2005, and suicide rates in some age groups have actually decreased
(9) . This is an important observation and, if verified in the final CDC data, deviates from our predictions. However, and perhaps most importantly, the 14% increase in the suicide rate among children ages 5 to 14 seen from 2003 to 2004 is not reversed in the preliminary 2005 data, and therefore remains a serious public health concern. This lack of decrease in suicide rates in younger age groups contrasts with a continuing decline in suicide rates among individuals over age 60, who experienced an ongoing increase in prescription rates during the same time period. These data continue to indicate that the FDA black box warning has not led to a reduction in youth suicide, which would be expected if the risk of antidepressant treatment outweighed the benefit. Although antidepressant prescriptions further decreased in this age cohort from 2004 to 2005, the real question is whether the most severely ill children who are at greatest risk of suicide are receiving treatment for their illness. It is possible that changes in the overall population-level antidepressant treatment rate may not accurately reflect the rate of antidepressant treatment among those children at greatest risk for suicide.
Drs. Wohlfarth, Boer, and van den Brink have done an excellent job synthesizing the results from our person-level findings from the VA study and our ecological studies and from those of Libby et al. Furthermore, they raise the important point of the limitations of randomized controlled trials that use placebo as a proxy for the absence of treatment. Our VA study
(4) showed that estimates of suicide attempts based on patients in randomized controlled trials dramatically underestimate the rate of suicide attempts in untreated patients. However, Drs. Wohlfarth, Boer, and van den Brink suggest that instead of resulting from the withdrawal of medication, the increase in suicidality may be due to the withdrawal of continued attention and caring that was previously provided in the context of pharmacologic treatment. Depression does indeed lift for some individuals when they are enrolled in clinical trials but provided only placebo (so-called placebo responders [
10,
11 ]), and the effectiveness of the supportive environment of psychotherapy suggests that a portion of the benefit of antidepressants could potentially be mediated by this continued attention. While we can think of no direct way to test this hypothesis using available data, the key issue remains that lack of attention to and/or denying treatment of children with clinical depression has serious public health consequences.
We thank our colleagues for continuing the dialogue and discussion of these important data and this important international issue. Families, clinicians, researchers, and suicide prevention advocates are all seeking to identify strategies to reduce the risk for suicide, and additional data are likely to provide a fuller understanding of this issue. We hope that the psychiatric research community will continue to work together to provide evidence-based answers to these important questions.