Alcohol dependence is a chronic relapsing disorder and second among the 10 most common medical causes of total years lost to disability
(1) . There are biological, genetic, and environmental factors contributing to the high rates of relapse. During the development of alcohol dependence, alterations of the brain reward system in cortical and limbic structures such as the amygdala, hippocampus, and ventral striatum have been observed and may convey the vulnerability for relapse in recovering alcohol-dependent subjects
(2) .
The amygdala is involved in the attachment of emotional valence to events (i.e., reward and punishment)
(3) and is in this way crucial for decision making and learning (e.g., reference
4 ). Furthermore, individuals with amygdala lesions fail to develop classically conditioned fear responses. Cocaine-dependent subjects who were also abusing alcohol displayed amygdala volume reductions that were connected to baseline measures of drug craving
(5) .
Amygdala volume reduction is an important anatomical correlate of alcohol use disorders and has previously been observed in individuals with alcohol dependence and in their offspring
(6 –
8) . The hippocampus and ventral striatum have also been reported to be similarly altered in heavy drinkers
(9), in adolescents with alcohol use disorders
(10,
11), and in adult alcoholics
(12 –
14) . Animal experiments implicate the amygdala, hippocampus, and nucleus accumbens in conditioned reinstatement of alcohol-seeking
(2) . Human measures of substance seeking, namely craving, have been observed to be associated with amygdala volume reduction in subjects dependent on cocaine and abusing alcohol
(5) . All three brain regions have been shown to be involved in various aspects of emotional memory function and in fear conditioning
(15 –
18) . Furthermore, all three brain regions have been shown to be important for the assessment of salience measures and valence (i.e., reward and aversion)
(3,
19) that are important for decision making and learning (e.g., reference
4 ). These observations raise the prospect that differences in brain volumes that process rewarding/aversive stimuli and are important for memory may be related to the relapse risk, in part, on the basis of increased alcohol craving.
We specifically assessed in alcohol-dependent subjects: 1) differences from healthy comparison subjects in the volume of the amygdala, hippocampus, and ventral striatum; 2) differences in alcohol craving; and 3) the association of these variables and subsequent relapse.
Method
Subjects and Instruments
Fifty-one alcohol-dependent patients (34 men and 17 women) who maintained abstinence for 1 week before scanning with magnetic resonance imaging (MRI) were included. They were group matched to 52 healthy comparison subjects (32 men and 20 women) on the basis of age and education and did not differ significantly for gender distribution (χ 2 =0.29, df, 1, p=0.59). All subjects were European and right-handed and provided written informed consent according to the Declaration of Helsinki. The Ethics Committee of the University of Heidelberg, Germany, approved the study.
The mean age of the patients was 41 years (SD=7, range=26–57) and 40 years (SD=8 years, range=26–63) for the comparison subjects. Standardized clinical assessment with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen
(20) and the Structured Clinical Interview for DSM-IV Personality Disorders
(21) was performed to exclude other active and lifetime axis I psychiatric disorders (and axis II disorders in healthy volunteers). Alcohol-dependent subjects fulfilled the diagnostic criteria for alcohol dependence according to ICD-10 and DSM-IV. The severity of alcohol dependence was assessed with the Alcohol Dependence Scale
(22), and the amount of lifetime alcohol intake was measured by the Lifetime Drinking History
(23) (
Table 1 ). The patients had no previous substance dependence or current substance abuse other than alcohol dependence (confirmed with random urine drug testing). The severity of depression was assessed with the Hamilton Depression Rating Scale
(24) and the German version of the Center for Epidemiologic Studies Depression Scale
(25) . Anxiety was measured with the State-Trait Anxiety Inventory
(26) and the severity of nicotine dependence by the Fagerström test
(27) . Exclusion criteria were hepatic impairment (e.g., liver cirrhosis) and clinically manifest neurological disorders (e.g., Wernicke-Korsakoff’s syndrome or alcoholic neuropathy) as determined by a senior neurologist.
Alcohol-dependent subjects participated in a 3-week inpatient detoxification program and were abstinent for at least 5 days (median=17 days, SD=14, range=5–96) before MRI was performed (control for the days of abstinence did not significantly correlate with any of the assessed brain volumes: all R<0.23, all p>0.12). Random breath and urine testing excluded current substance or alcohol abuse. All subjects were medication free (including benzodiazepines, chlormethiazole, acamprosate, naltrexone, etc.) for at least 1 week before testing.
Before scanning, craving for alcohol was measured with a visual analogue craving scale (1–100). After discharge, the patients were seen biweekly for another 6 months, and alcohol consumption was recorded with the Form 90
(28) . In accordance with standard clinical trials
(29), relapse was defined as a consumption of more than 60 grams of alcohol in men or more than 40 grams of alcohol in women during the assessment period. By using these criteria, 31 patients relapsed and 17 patients stayed abstinent. For three patients, we could not clarify the relapse status. Random alcohol blood and breath tests were performed, levels of carbohydrate-deficient transferrin and gamma-glutamyltransferase were evaluated, and relatives or members of their self-help group were contacted to verify the patients’ abstinence. Besides alcohol craving, no other alcohol-related factors such as the amount of alcohol consumption over the lifetime, duration of alcohol dependence, age of onset, and number of cigarettes smoked per day were associated with the volume of the assessed brain regions (all R<–0.22, all p>0.13).
Neuroimaging
MRI image acquisition
Scanning was performed by using a 1.5 T clinical whole-body MRI (Magnetom VISION, Siemens, Erlangen, Germany) equipped with a standard quadrature head coil. The automatic Siemens Multi-Angle Projection shim was used for shimming. A morphological three-dimensional T 1 -weighted magnetization-prepared rapid gradient echo image data set (1×1×1 mm=voxel size, field of view=256 mm, 162 slices, TR=11.4 msec, TE=4.4 msec, α=12°) covering the whole head was acquired for anatomical study.
Morphometric analysis
Gray-white matter segmentation was performed on each T
1 -weighted, positionally normalized, three-dimensional coronal scan by using a semiautomated intensity contour mapping algorithm for cerebral exterior definition and signal intensity histogram distributions for demarcation of gray-white matter borders
(30) .
The segmentation method applied followed prior applications
(5) : all structures were segmented by using a contour line and manual editing. To distinguish the amygdala and hippocampus, sulci lines were drawn in the sagittal views, and the anterior tip of the hippocampus was separated from the ventral and posterior border of the amygdala. Using lateral and sagittal views, one can distinguish and trace this border of the amygdala, which was usually enhanced by the anterior end of the temporal horn of the inferior lateral ventricle. In cross-reference, corresponding axial views helped to identify this border. In the coronal view, the saw-tooth pattern of the hippocampus was identified and traced. The ventral striatum was identified at the inferior junction between the head of the caudate and the putamen. It was delimited superiorly by a line connecting the inferior corner of the lateral ventricle and the most inferior point of the internal capsule abutting the ventral striatum and laterally by a vertical line passing from the latter point. The total volume resulted from the addition of the left and right volumes.
Segmentation was performed by two bachelor’s level magnetic resonance technicians who were blind to subject diagnosis and group status and with a randomly ordered sequence of subjects. Intrarater and interrater reliabilities were assessed through percent common voxel assignments
(31) . Reliabilities were all between 85.9 and 90.1. These values are consistent with intrarater and interrater reliabilities reported in previous studies (e.g., references
5 and
31 ).
To rule out gross volumetric effects, total head circumference was calculated for all subjects. Group differences were not significant for this measure (all p>0.05).
For topological analysis, subsequent relapsing and abstaining alcohol-dependent subjects were registered to a reference brain that was separate from either cohort. Amygdala and hippocampus probabilities for each group were calculated on a voxel-by-voxel basis with the aligned data. Iso-surfaces for the probability 0.5 were created for each group separately
(5) . Three-dimensional visualization of these surfaces was used to search for systematic differences in the topology of the amygdala between groups.
Statistical Analysis
For a comparison of brain volumes between healthy comparison subjects, relapsers, and abstainers, a multivariate analysis of variance (MANOVA) was used with sex and morphometry rater as covariates and the post hoc test was corrected with the least significant difference. Since craving data were not normally distributed, the Mann-Whitney U test was used for the comparison between relapsers, abstainers, and comparison subjects, and the significance threshold was Bonferroni-corrected for multiple comparisons (p=0.05/3=0.0166). Amygdala volume and craving were correlated using Spearman’s rho (p<0.05, two-tailed).
Results
Group Comparison
Alcohol-dependent patients displayed significantly more liver dysfunction and negative mood states in relation to healthy comparison subjects (
Table 1 ); however, the groups of subsequently relapsing versus abstaining patients did not differ significantly from each other with respect to these clinical variables.
Volumes
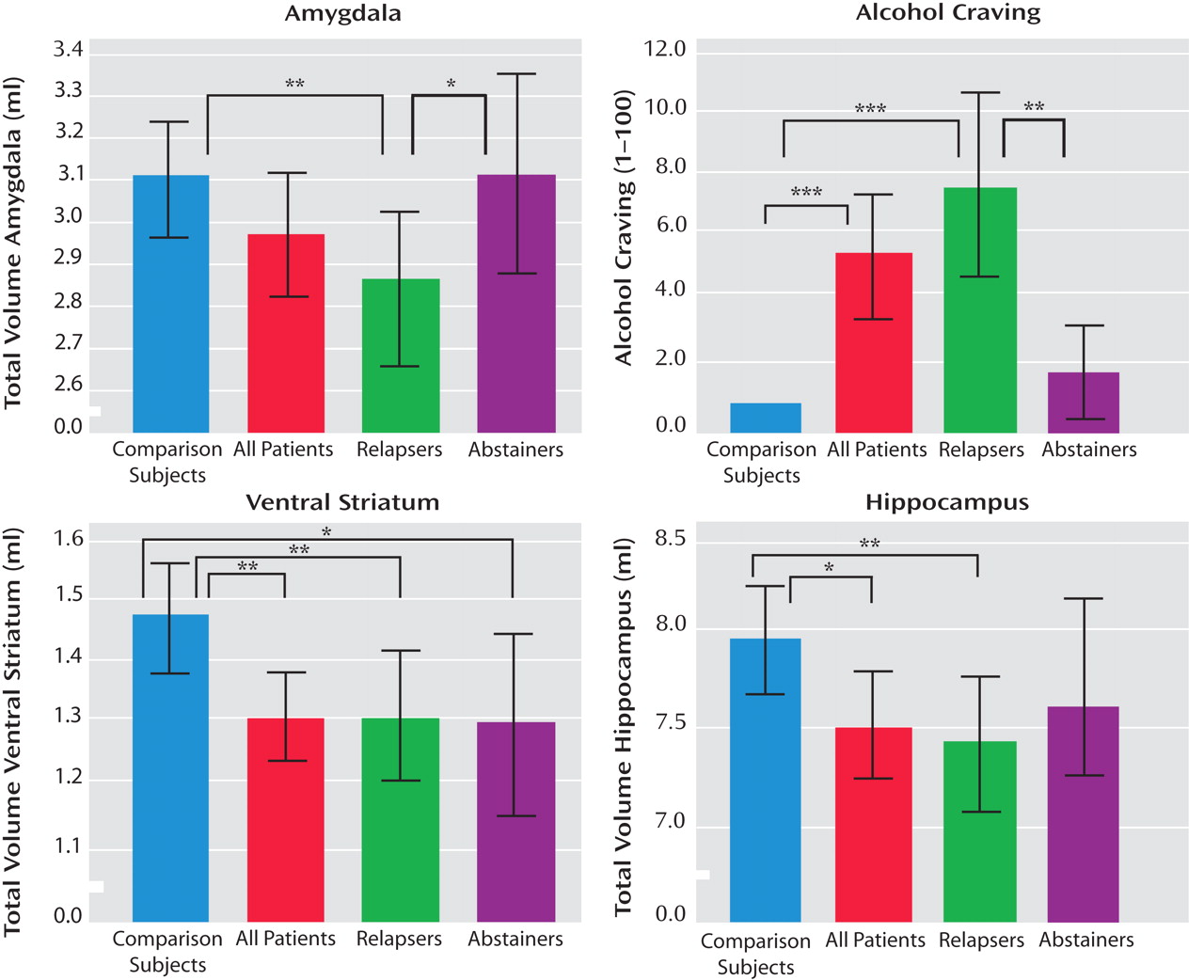
In alcohol-dependent subjects in relation to healthy comparison subjects, we observed a significant volume reduction in the hippocampus (T=2.49, p<0.02) and the ventral striatum (T=2.87, p=0.005) and a nonsignificant reduction in the amygdala (T=1.45, p=0.15). Considering the relapse status during the 6-month follow-up period, a MANOVA revealed a significant effect for the group factor (comparison subjects, relapsers, and abstainers) (Wilks’s lambda=3.57, df=6, p=0.002), with significant effects for all three regions (the amygdala: p=0.007, the hippocampus: p=0.008, and the ventral striatum: p=0.003). This result remained statistically significant even when control was added for total gray matter volume (Wilks’s lambda=2.43, df=6, p<0.03), with significant effects for all three brain regions: the amygdala: p<0.03, the hippocampus: p<0.05, and the ventral striatum: p<0.04). Post hoc tests revealed that the amygdala volume was significantly reduced in relapsers compared to both abstainers (p<0.05) and comparison subjects (p=0.002), but there was no significant difference between abstainers and comparison subjects (p=0.99) (
Figure 1 ). Ventral striatal volume was reduced in relapsers (p=0.004) and abstainers (p<0.02) in relation to healthy comparison subjects, but no significant difference was observed between abstainers and relapsers (p=0.94) (
Figure 1 ). For the hippocampal volume, we found a significant difference between relapsers and comparison subjects (p=0.004) but no significant differences between abstainers and comparison subjects (p=0.26) and abstainers and relapsers (p=0.25) (
Figure 1 ).
Topological data
The average surface of the amygdala for the relapsers lay slightly above and in front of the surface of the abstainers in a continuous fashion from an anterior-inferior position to an anterior-superior position. The larger extent of the amygdala observed in the abstainers was observed along both the inferior and posterior-superior aspects of the amygdala. The maximum extent differences between relapsing and abstaining alcohol-dependent subjects at the back of the amygdala were 3 mm to 4 mm. This area represents regions in which abstinent individuals have amygdala tissue but the relapsing group does not. Using the atlas of Mai et al.
(32) for reference, these pattern differences encompass nuclei from the basolateral group (i.e., lateral nucleus) in the portion of the amygdala adjacent to the subiculum. Although no absolute group difference in hippocampal volume between abstainers and relapsers was found, findings within the hippocampus (i.e., subiculum) parallel those with the amygdala: the hippocampus of subsequent abstinent alcohol-dependent subjects extended about 3 mm to 4 mm beyond those of subsequent relapsing alcohol-dependent subjects along its inferior and posterior-superior aspects, encompassing components of the subiculum.
Craving
Alcohol-dependent subjects reported a significantly stronger craving for alcohol than healthy comparison subjects (Mann-Whitney U=636, p<0.0001) (
Figure 1 ). Dividing alcohol-dependent patients into subsequent relapsers and abstainers, subsequent relapsers showed a significantly stronger craving compared to subsequent abstainers (Mann-Whitney U=123.5, p=0.003) and to comparison subjects (Mann-Whitney U=230, p<0.0001). There was no difference between subsequently abstaining patients and healthy comparison subjects (Mann-Whitney U=368, p=0.38) (
Figure 1 ).
Association Analysis
Smaller amygdala volume was associated with increased alcohol craving in alcohol-dependent subjects (R=–0.36, p<0.02) but not in comparison subjects (R=–0.27, p<0.06). Separating relapsers and abstainers, a significant association between amygdala volume and craving was found in relapsers (R=–0.38, p<0.04) but not in abstainers (R=–0.17, p=0.51).
Discussion
Our study showed for the first time that amygdala volume reduction, located mainly in the basolateral amygdala, and alcohol craving differentiated between subsequently relapsing and abstaining alcohol-dependent subjects.
The amygdala comprises more than a dozen subnuclei, which by a topological perspective can be categorized into three general divisions: basolateral, corticomedial, and central nuclear/anterior area
(33) . The smaller extension of amygdala volume in relapsing compared with abstinent alcohol-dependent subjects was observed along both inferior and posterior-superior aspects of the amygdala encompassing subnuclei from the basolateral group. Studies in rodents have shown that the basolateral amygdala is critical to learning representations linking stimuli to the incentive values of outcomes
(4,
34) . In primates, changes in the positive and negative values of cues modulate neural activity in the amygdala, and this modulation occurs fast enough to account for learning
(35) . In human studies with functional MRI, it has been shown that amygdala activation is correlated with 1) assessment of positive and negative stimuli
(36), 2) expectancies of rewards and losses
(19), 3) valuation of reward representations accessible to predictive cues
(37), and 4) processing of alcohol-associated cues in alcohol-dependent subjects
(38) .
Animals with basolateral amygdala lesions lack response flexibility during unconditioned stimulus devaluation
(39) and display an increase in impulsive choice
(4) . Alcohol-dependent subjects with amygdala volume alterations display decision-making deficits in a simulated gambling task, suggesting that decision-making deficits in everyday life of abstinent alcohol-dependent subjects may reflect an alteration in brain structure
(7) . These factors may contribute to poor treatment outcome in alcohol-dependent subjects with amygdala volume differences.
Our study does not address the question of whether amygdala volume reduction is a cause or consequence of chronic alcohol intake. Overall reductions in brain volume have often been attributed to ethanol’s neurotoxic effects, but other environmental effects and genetic factors may also play a role
(40) . Fein and coworkers
(7) found amygdala reduction in long-term abstinent alcohol-dependent subjects (on average 7.1 per year) with no association between amygdala volume and duration of abstinence. Also, in our data, results were not affected when we controlled for the duration of abstinence before scanning. This finding may indicate that amygdala volume reduction is a trait rather than a state marker and does not recover with abstinence. Hill
(6) and Benegal and coworkers
(8) observed amygdala volume reduction in adolescent and young adult offspring from families with a history of alcohol dependence, suggesting that reduced amygdala volume may—at least in part—be present even before excessive alcohol intake starts and may reflect a preexisting condition that predisposes to alcohol dependence and severe patterns of consumption. Assuming that the difference in brain volumes is a trait marker, follow-up studies might compare 1) alcohol-dependent subjects who tried several times to stay abstinent but failed with 2) alcohol-dependent subjects who have been abstinent long-term and healthy comparison subjects.
Volume reductions of the ventral striatum and subiculum region of the hippocampus were found in this study in all alcohol-dependent subjects in relation to healthy comparison subjects, but there was no difference between subsequent relapsing and abstaining patients. This finding emphasizes that volume reduction of the ventral striatum and hippocampus is a common finding in alcohol-dependent subjects, which, however, did not discriminate between subsequent relapsers and abstainers in our study.
In summary, this study suggests that amygdala volume reduction is associated with alcohol craving and an increased prospective relapse risk.