Obsessive-compulsive disorder (OCD) is a seriously debilitating neuropsychiatric disorder with a lifetime prevalence rate of 2%–3% in the general population
(1) . Abnormal feedback loops within cortical-striatal-thalamic-cortical circuits have been hypothesized to play a key role in the pathophysiology of OCD
(2) . Specifically, a defect in the ability of the basal ganglia and thalamus to provide sensory gating functions by filtering and suppressing inputs from cortical regions has been considered important to OCD phenomenology and/or effective pharmacotherapy
(3) . Moreover, structural abnormalities in cortical regions comprising basal ganglia-thalamocortical circuits
(4), including the orbitofrontal cortex
(5,
6) and the anterior cingulate
(7,
8), have also been implicated in neurobiological models of OCD.
Relatively automated techniques for examining the brain in vivo, such as voxel-based morphometry, permit investigators to quantitatively and comprehensively examine brain structural differences between patients with neuropsychiatric disorders and healthy volunteers. A potentially important advantage of this relatively automated approach is that abnormalities may be identified with greater reliability compared to volumetric region-of-interest approaches and may provide additional information beyond regional volumetry. A number of studies in adults have used voxel-based morphometry to examine the neurobiology of OCD, but findings have been inconsistent. Pujol et al.
(5) reported less gray matter volume in the medial frontal gyrus, the medial orbitofrontal cortex, and the left insular-opercular region but observed more gray matter bilaterally in the ventral putamen and the anterior cerebellum among patients. Valente et al.
(9) identified more gray matter in patients in posterior orbitofrontal and parahippocampal regions as well as less anterior cingulate gray matter. In a study that assessed gray matter density, Kim et al.
(10) reported greater density in patients compared to healthy volunteers in the left orbitofrontal, left superior temporal, left inferior parietal, left thalamus, right insula, right middle temporal, right inferior occipital, and bilateral hypothalamus. In contrast, less density in patients was observed in the left cerebellum and left cuneus. A potential limitation of prior studies, however, is the inclusion of patients with considerable illness duration and diagnostic comorbidity. Moreover, the majority of patients in prior studies have been treated previously with psychotropic medications, which may be associated with changes in gray matter volume in OCD
(11) .
Subjects and Methods
Subjects
Thirty-seven psychotropic drug-naive pediatric outpatients with OCD and 26 healthy comparison subjects participated in this study (see
Table 1 ). All patients were recruited through the child psychiatry outpatient clinic at Wayne State University School of Medicine in Detroit. Patients were diagnosed with DSM-IV criteria and the Schedule for Affective Disorders and Schizophrenia for School Age Children—Present and Lifetime (K-SADS-PL)
(13) versions. All subjects and their parents were interviewed by a board-certified child and adolescent psychiatrist (D.R.R.). Nine patients had comorbid anxiety disorders, three had dysthymia, two had oppositional defiant disorder, two had attention deficit disorder without hyperactivity, one had attention deficit disorder with hyperactivity, two had trichotillomania, and 23 had OCD as their only diagnosis. Mean age at onset was 9.4 years (SD=3.1). Fifteen patients and 17 healthy volunteers in this study had participated in our prior volumetric study of brain structure
(7), and five patients and eight healthy volunteers had participated in our prior study of amygdala volume changes in association with selective serotonin reuptake inhibitor (SSRI) treatment
(11) .
There was no history of psychiatric illness in the healthy comparison subjects or in any of their first-degree relatives. Exclusion criteria for patients and healthy comparison subjects included a lifetime history of unipolar or bipolar disorder, psychosis, eating disorders, substance abuse or dependence, Sydenham’s chorea, Tourette’s syndrome and other tic-related conditions, conduct disorder, significantly debilitating medical or neurological conditions, pervasive developmental disorders, mental retardation, or learning disorders. The child’s parents (and the child, when appropriate) served as informants for detailed diagnostic assessments, including the K-SADS-PL. The study was approved by the institutional review board, legal guardians provided written informed consent, and all subjects provided written assent.
Clinical Assessments
All subjects were administered the Children’s Yale-Brown Obsessive Compulsive Scale
(14) to assess OCD symptom severity, although one patient was excluded from analyses examining the clinical correlates of brain structural abnormalities owing to an outlying low value (>2 SDs). The 17-item Hamilton Depression Rating Scale (HAM-D) measured severity of depression, and the Hamilton Anxiety Rating Scale (HAM-A) measured severity of anxiety. Tic severity was measured with the Yale Global Tic Severity Scale
(15) . We classified patients according to the five clinical dimensions defined by Mataix-Cols and colleagues
(16) . Using these criteria, we found that patients’ predominant obsessions/compulsions were as follows (numbers in parentheses): symmetry/ordering (N=2), hoarding (N=1), contamination/cleaning (N=13), aggressive/checking (N=11), and sexual/religious (N=1). Classifications could not be made for nine individuals.
Neuropsychological Assessment
A brief neuropsychological examination that included measures of general intelligence
(17), attention
(18), and motor coordination
(19) was administered to patients and healthy volunteers to investigate possible group differences in functioning that might account for the observed findings.
Magnetic Resonance Imaging (MRI) Procedures
MRI examinations were conducted at the Children’s Hospital of Michigan Imaging Center with image acquisition methods described previously
(7) . Briefly, MRIs were acquired in the coronal plane using a three-dimensional spoiled gradient echo pulse sequence with a 40° flip angle, a 25-msec repetition time, and a 5-msec echo time on a 1.5 T whole-body superconducting imaging system (General Electric, Milwaukee). This sequence produced 124 contiguous coronal slices (slice thickness=1.5 mm) through the whole head with a nominal in-plane resolution of 0.94 mm × 0.94 mm in a 256×256 matrix. Axial proton density and T
2 -weighted images were obtained to exclude visually detectable structural abnormalities on MRI scans.
Image Analysis Procedures
Voxel-based morphometry image analysis was performed on a computer workstation with MATLAB 6.5.1 and SPM2 software (Wellcome Department of Cognitive Neurology, London) with methods similar to those described previously
(5) . We used the following steps of the optimized voxel-based morphometry routine in SPM2 to enhance sensitivity by creating a study-specific template and then reincorporating volume information lost during the transformation process. In this approach, the individual MRI scans were spatially normalized from native space to the Montreal Neurological Institute 152-image template with a 12-parameter affine transformation with 16 nonlinear iterations and a 25-mm nonlinear basis function cutoff. Normalized images were then segmented with signal intensity and prior probability information. The normalized segmented images were then smoothed with an 8-mm isotropic Gaussian kernel and averaged to create study-specific gray matter, white matter, and CSF templates. The original (untransformed) subject MRI scans were segmented in native space. Segmented images were spatially normalized to a study-specific template. Intensity modulation was performed on the optimally normalized, segmented gray matter MRI scans by increasing or reducing voxel intensity, depending on whether the surrounding voxels were compressed or expanded in the normalization process. Modulated gray matter images were smoothed with an 8-mm full width at half maximum isotropic Gaussian kernel before analysis with voxelwise group comparisons.
Manual Region-of-Interest Measurements
We employed a manual region-of-interest approach to confirm structural alterations in the putamen and orbital frontal cortex identified in SPM2. In addition, we measured the anterior cingulate given its importance to neurobiological models of OCD. Regions were measured with previously published delineation criteria
(7,
20) . All gray matter region-of-interest measurements were computed using the MEDx (2004, v3.43, Sensor Systems, Virginia) software program. Because one of the limiting sulci required for measurement of the orbital frontal region (i.e., the anterior horizontal ramus) was not present in every hemisphere
(20), orbital frontal volumes could not be computed in the right hemisphere for three patients and four healthy volunteers and in the left hemisphere for four patients and one healthy volunteer. MRI scans were aligned along the anterior and posterior commissures for standardization across subjects. Scans were mixed together randomly, and no identifying information was available to the operator from the scan. Intracranial volumes were computed with National Institutes of Health images and included all brain gray and white matter, including the frontal, parietal, temporal, and occipital lobes, but excluded the cerebellum and brainstem. All measurements were completed by an operator who was blind to group membership. Intraclass correlations for two raters ranged from 0.87 to 0.99 for the right and left putamen, orbital frontal cortex, anterior cingulate, and intracranial volume. As an exploratory aim, we compared volumes obtained using our region-of-interest methodology with predefined regions of interest in SPM2 using the Anatomical Automatic Labeling package
(21) .
Data Analysis
Group differences in demographic variables were examined with independent t tests. Chi-square tests were used to examine differences in joint classifications of discrete variables. Group comparisons were performed using analysis of covariance (ANCOVA) in SPM2, with diagnostic group as a fixed factor and intracranial volume as a covariate. Two planned contrasts were examined: patients > comparison subjects and patients < comparison subjects. For maxima falling within a priori regions of interest (the putamen, thalamus, orbital frontal cortex, and anterior cingulate cortex), the threshold for statistical significance was set to p<0.005 (uncorrected), with a minimum of 200 contiguous voxels required. For comparison purposes, other regions across the entire brain that were not predicted a priori are reported at a minimum of 200 voxels attaining p<0.005 (uncorrected), but statistical significance was assessed with a strict family-wise error threshold of p<0.05 (corrected). Post hoc analyses examined the subgroups of patients with predominant contamination/cleaning (N=13) or aggressive/checking (N=11) symptoms (the only subgroups large enough for analyses) compared to healthy volunteers and each other.
Volumetric data were analyzed with independent group t tests and ANCOVA where group (patient versus healthy volunteer) was the between-subjects factor and age and intracranial volume served as statistical covariates. Distributions of manually derived brain volumes were examined for normality, skewness, and kurtosis. Outlying values (defined as greater than 2 SDs from the mean) were excluded from analysis. This affected data for the following number of manual region-of-interest measurements (patients, comparison subjects): right putamen (1, 1), left putamen (1, 1), right orbital frontal cortex (0, 1), left orbital frontal cortex (1, 0), right anterior cingulate (2, 1), and left anterior cingulate (1, 0). Associations between continuous variables, including subject values of modulated intensity (i.e., volume) for significant clusters, were output from SPM2 (with the volume of interest tool) for analysis in SPSS (v11.5) (SPSS, Chicago) with Pearson’s product-moment correlations. The alpha level was set to 0.05 for region-of-interest and correlational analyses.
Results
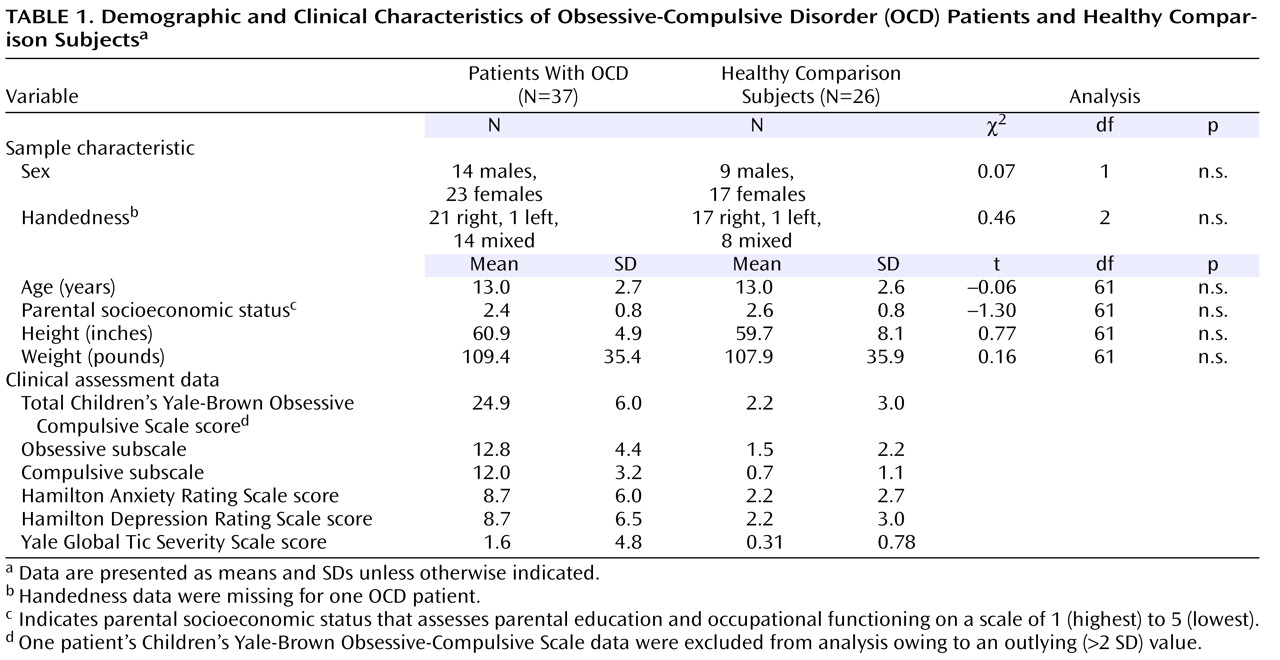
Demographic characteristics and clinical assessment data for psychotropic drug-naive patients with OCD and healthy comparison subjects are provided in
Table 1 . Patients and comparison subjects did not differ significantly in distributions of age, sex, height, weight, parental social class, or handedness. In addition, there were no significant group differences in measures of general intelligence, attention, or motor coordination. Mean (SD) gray matter volume derived from SPM2 for patients was 776 cm
3 (SD=69) and for healthy volunteers was 747 cm
3 (SD=68). Mean white matter volume derived from SPM2 for patients was 401 cm
3 (SD=48) and for healthy volunteers was 377 cm
3 (SD=44). Neither gray nor white matter volumes differed significantly between groups.
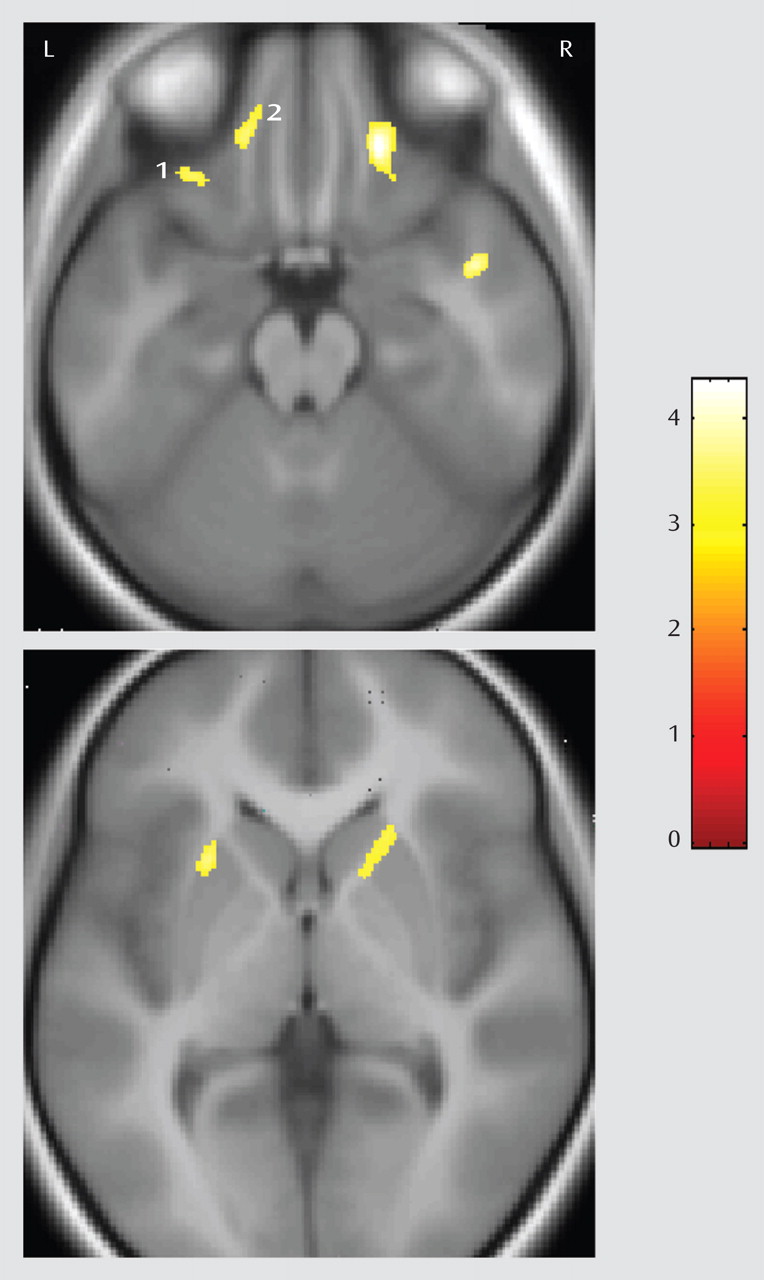
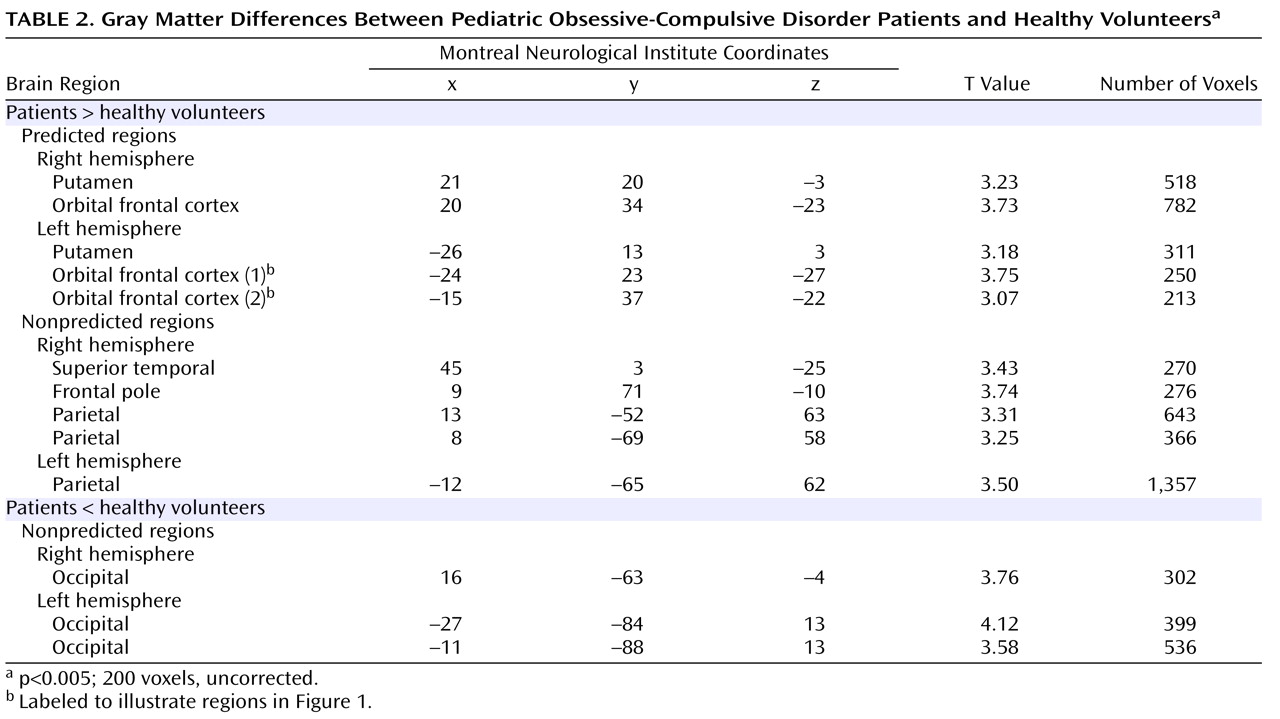
Pediatric OCD patients had significantly (p<0.005, uncorrected; 200 voxels) more gray matter in regions predicted to differ a priori between groups, including the right and left putamen and orbital frontal cortex (see
Figure 1 ). Montreal Neurological Institute coordinates for these regions are provided in
Table 2 . These results remained statistically significant when we excluded patients with comorbid diagnoses from analysis. Post hoc volume of interest analyses indicated that subgroups of patients with contamination/cleaning or aggressive/checking symptoms had significantly more gray matter in the right and left putamen and orbital frontal cortex compared to healthy volunteers (all p<0.05). In addition, patients with contamination/cleaning symptoms did not differ significantly from patients with aggressive/checking symptoms in either right or left putamen and orbital frontal cortex volumes.
Investigation of the clinical correlates of abnormalities in patients revealed that higher OCD symptom severity correlated significantly with greater left putamen (r=0.34, df=36, p=0.04) and right orbital frontal cortex (r=0.36, df=36, p=0.03) volume. Neither right or left putamen or orbital frontal cortex volumes correlated significantly with anxiety or depression scores or with age of onset. There was a significant positive correlation (r=0.77, df=11, p=0.006) between greater Children’s Yale-Brown Obsessive Compulsive Scale scores and more left orbital frontal cortex gray matter (see
Figure 1, region 2) in the subgroup of patients with predominant aggressive/checking symptoms. Among this subgroup of patients, there were tendencies for greater Children’s Yale-Brown Obsessive Compulsive Scale score severity to be associated with more left putamen gray matter (r=0.60, df=11, p=0.051) and right orbital frontal gray matter (r=0.54, df=11, p=0.08). In contrast, there were no tendencies for these correlations to be significant (all p>0.10) among the subgroup of patients with predominant contamination/cleaning symptoms.
Exploratory analyses conducted across the entire brain revealed several additional regions where more gray matter was observed in patients compared to healthy volunteers, including the frontal pole, superior temporal gyrus, and parietal lobe (
Table 2 ). In addition, several areas of less gray matter in the occipital cortex, which were not predicted a priori, were observed in patients compared to healthy volunteers in both the right and left hemispheres (
Table 2 ).
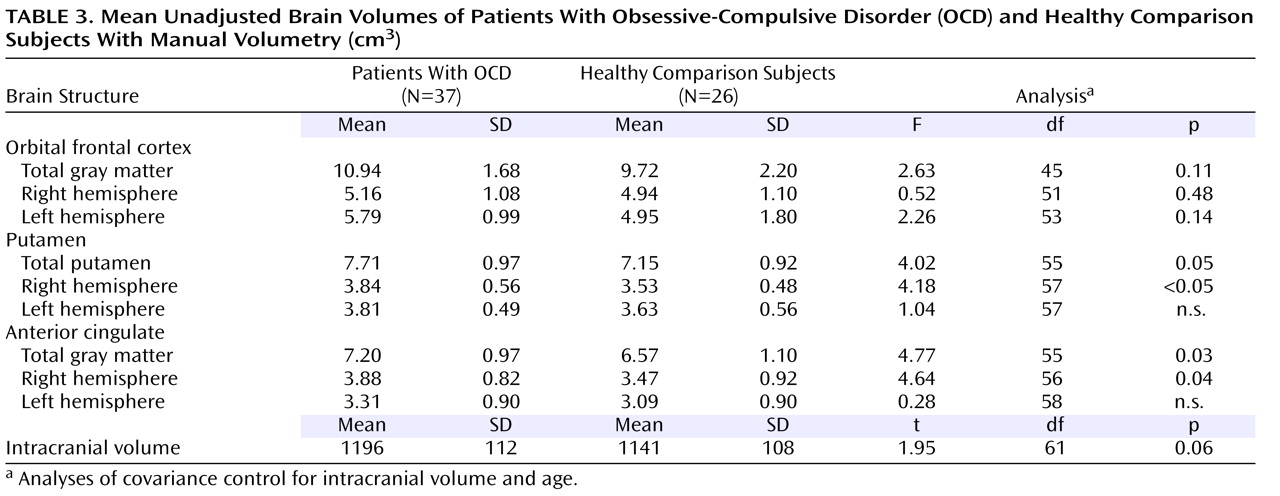
Unadjusted means and standard deviations for manual region-of-interest measurements of the orbital frontal cortex, putamen, and anterior cingulate are presented in
Table 3 . Independent group t tests revealed significantly more left orbital frontal cortex, right putamen, and right anterior cingulate gray matter volume in patients compared to healthy volunteers (p<0.05). After control was added for the effects of age and intracranial volume, however, group differences were no longer statistically significant for the left orbital frontal cortex but remained significant for right putamen and right anterior cingulate gray matter volume (see
Table 3 ).
As an exploratory aim, we examined the relationship between brain volumes derived from SPM2 and those obtained using manual volumetric methods. Total putamen volume obtained using the Anatomical Automatic Labeling package correlated significantly with total putamen volume derived from manual volumetry (r=0.30, df=59, p<0.03). In addition, measurements of the superior (r=0.33, df=49, p=0.02) and middle (r=0.30, df=49, p<0.04) parts of the orbital surface (where gray matter structural alterations appeared most pronounced in the voxelwise analysis) correlated significantly with the manually drawn orbital frontal cortex volumes.
Discussion
Although several voxel-based morphometry studies have been conducted in OCD
(5,
9,
10), patient samples included adults. Because OCD may have pediatric onset in as many as 80% of cases, investigation of its neurobiology may be facilitated by studying the illness in childhood, which decreases the likelihood of potentially confounding variables. Our results thus extend prior work in this area by demonstrating brain structural abnormalities in regions comprising cortical-striatal-thalamic-cortical loops early in the course of illness without the potential confound of prior SSRI usage. Given that administration of SSRIs has been linked to gray matter structural changes in OCD
(11), it is conceivable that prior MRI studies, which used patients treated previously with SSRIs, may have been influenced by medication exposure.
Prior structural neuroimaging studies investigating the orbital frontal cortex in OCD have yielded mixed findings. Our voxel-based morphometry findings of more orbital frontal cortex gray matter in pediatric patients converges with several prior voxel-based morphometry studies conducted in adults with OCD
(9,
10) but is contradictory to two region-of-interest studies
(6,
22) and a voxel-based morphometry study
(5) that reported less orbital frontal volume in adult patients. As acknowledged previously, an important methodologic limitation in our prior study
(6) was the lack of segmentation in the orbital frontal lobe, thus making it difficult to directly compare the present results with our prior study. Moreover, our prior study as well as others
(5) comprised adults treated previously with SSRIs in contrast to the use of psychotropic drug-naive children and adolescents in the present study. In addition, differences among studies could reflect the use of voxel-based morphometry versus manual region-of-interest methods. Specifically, voxel-based morphometry provides information about particular areas within a structure that are maximally different between groups, whereas region-of-interest approaches reflect volumetric differences across an entire brain structure. Thus, abnormalities localized to discrete parts of the orbital frontal cortex observed with voxel-based morphometry may be difficult to identify using a region-of-interest approach because such effects may be attenuated when the entire volume is examined. This may partially explain why we did not observe identical results with voxel-based morphometry and manual volumetry for the orbital frontal cortex, although the results from these two approaches are strongly consistent with one another, at least for the left hemisphere.
The finding of greater orbital frontal gray matter volume in patients with OCD compared to healthy volunteers in our study converges with the results of functional neuroimaging studies. In addition, treatment studies reported decreases in orbital frontal brain activity following SSRI pharmacotherapy, which correlate with improvement in symptoms
(23), and that orbital frontal brain metabolism predicted subsequent response to SSRI pharmacotherapy
(24) . Increased orbital frontal activity has been observed during positron emission tomography (PET)
(25) studies of symptom provocation in OCD, thus supporting a role for this brain region in phenomenology. Moreover, there is neuropsychological evidence that patients with OCD perform poorly on tasks believed to be mediated, at least in part, by the orbital frontal lobe, including olfaction
(26) .
In our study, abnormalities appeared more pronounced in lateral in contrast to medial (i.e., gyrus rectus) orbital frontal regions among patients with OCD compared to healthy volunteers. Recent theoretical and empirical work highlights a double dissociation between medial and lateral orbital frontal systems, which may have relevance for the findings reported herein. Hurliman et al.
(27) provide functional neuroimaging evidence for two ventral frontal feedback systems, including a lateral system, which is specialized for sensory/exteroceptive processing, and a medial system, which plays a role in processing visceroappetitive and interoceptive information. Moreover, in an elegant review, Elliott et al.
(28) proposed a double dissociation between medial and lateral orbital frontal regions. Specifically, they provide neuroimaging evidence that the medial orbital frontal region plays a role in monitoring the relationship among stimuli under changing environmental exigencies in contrast to the lateral orbital region, which plays a role in the interruption of behaviors based on prior reward values of stimulus-response associations. Our findings may thus have relevance for OCD patients’ difficulty discontinuing their compulsions given how rewarding these compulsions are in attenuating anxiety.
In their neurobiological models, Baxter et al.
(29) and Insel
(2) emphasized a role for the orbital cortex in OCD. In particular, Baxter and colleagues suggested that a defect in basal ganglia functioning could lead to deficient “filtering” of orbital frontal cortex “worry” inputs. The threshold of this system in OCD may be abnormally low due to increased orbital frontal activity or lowered basal ganglia filtering. Such circuits presumably have an evolutionary basis and may serve to direct attention for needed action involving OCD symptoms (e.g., contamination). An imbalance in this system could represent the neuroanatomic substrate for being overwhelmed by internal cues frequently reported by patients, especially when goal-directed behavior is required
(30) . Moreover, we previously suggested that the orbital frontal cortex is part of a ventral “paleocortical” tendency in brain development
(31) that may have particular relevance for the phenomenology of OCD
(6) . For example, in OCD, a disruption in regions comprising the paleocortical tendency may serve to restrict motor control in service of “adaptive constraints of self-preservation”
(32) .
Our data also provide strong evidence that structural alterations in the putamen play a role in the pathophysiology of OCD. Specifically, we found evidence for larger putamen volume (bilaterally) in pediatric OCD patients compared to healthy volunteers, which were replicated for the right hemisphere with a manual region-of-interest approach. Our findings converge with a voxel-based morphometry study by Pujol et al.
(5), who reported an increase in gray matter volume bilaterally in the putamen in adults with OCD compared to healthy volunteers. Moreover, our findings are consistent with functional imaging data reporting hypermetabolism in the putamen among patients with OCD
(33) . Several volumetric investigations, however, reported either smaller
(34) or no differences
(7) in putamen volume in pediatric OCD compared to healthy volunteers with a region-of-interest approach. It is possible that sampling differences between the present study and prior studies may at least partly account for different findings. For example, there is some evidence that, at least for a subset of children, obsessive-compulsive symptoms may arise from an interaction of antibodies to group A beta-hemolytic streptococcal infection with neurons of the basal ganglia, particularly in the putamen
(35) .
The putamen has been hypothesized to play an important role in motor and cognitive control, which has particular relevance for neurobiological models of OCD. In that regard, our data are consistent with neuropsychological and symptom data suggesting that a defect in the putamen contributes to the pathogenesis of OCD. In a neuropsychological investigation, Rauch et al.
(36) reported that healthy volunteers activated the right and left striatum during an implicit sequence learning task with subsequent work demonstrating that these effects are most pronounced in the right putamen
(37) . In contrast, patients with OCD did not activate corticostriatal regions but instead showed activation in medial temporal lobe regions typically associated with the processing of explicit (or conscious) information processing. In another neuropsychological study, greater decreases in metabolic activity in the right putamen after SSRI pharmacotherapy in OCD patients correlated significantly with greater improvements in the Rey Osterrieth Complex Figure Test
(38) . Moreover, our finding that larger putamen volume correlated significantly with greater symptom severity among patients converges with the results of a functional imaging study
(39) that indicated that higher left putamen brain metabolism was associated with OCD symptom severity.
Although we did not observe more anterior cingulate gray matter in this cohort of psychotropic drug-naive patients with OCD using voxel-based morphometry at the specified significance threshold, these effects were evident using regional morphometry, which is consistent with our prior reports
(7,
8) but inconsistent with a voxel-based morphometry study that reported less gray matter in previously treated adult patients
(9) . A possible explanation for the discrepant findings between voxel-based morphometry and manual volumetry in our study may be related to our use of anatomically relevant “parcellation units,” which are based on the sulcal anatomy, whereas SPM is focused on discrete regions within the anterior cingulate that are maximally different between groups. It is therefore plausible that anterior cingulate abnormalities are more widely distributed throughout the structure. Although this study cannot be considered an independent replication of our prior work
(7) given the overlap in samples, the finding of more anterior cingulate gray matter in patients is consistent with the results of functional brain imaging studies reporting hypermetabolism in this area during symptom provocation
(40) and reductions in metabolic activity following treatment
(41) .
The assessment of clinical subtypes in OCD may contribute to our understanding of the neurobiological mechanisms underlying this heterogeneous disorder. In the present study, patients with predominant aggressive/checking compulsions had larger putamen volumes compared to healthy volunteers. This finding converges with a recent functional MRI symptom provocation study by Mataix-Cols et al.
(42), who reported greater activation in the putamen/globus pallidus among patients with predominant checking compulsions compared to controls. It is important to note, however, that in our study, patients with predominant contamination/cleaning symptoms also had larger putamen volumes compared to healthy volunteers, suggesting nonspecificity of findings. Investigation of other symptom dimensions in our study was not possible because of the low frequency of patients in these subgroups. Nonetheless, our findings support the hypothesis that a defect in the putamen plays a role in the pathogenesis of OCD, at least for the two symptom dimensions investigated.
We also observed more gray matter in patients compared to healthy volunteers in several other brain regions not hypothesized to differ between groups a priori, including the frontal pole, superior temporal gyrus, and parietal lobule. Because none of these regions was predicted to differ a priori between groups and none survived strict correction for multiple comparisons (family-wise error>0.05), these results should be interpreted with caution. Nonetheless, findings of parietal lobe structural alterations among OCD patients in our study are consistent with a growing body of evidence implicating parietal lobe dysfunction in the pathophysiology of OCD
(38,
43) and a PET study demonstrating that cerebral glucose metabolism in the parietal region correlated with OCD severity
(39) . In addition, findings of less gray matter in patients compared to healthy volunteers did not occur in regions predicted to differ significantly between groups a priori and, likewise, should be interpreted with caution.
In sum, the use of complementary techniques in the present study provides strong evidence for dysfunction of the orbital frontal cortex and putamen in the pathophysiology of OCD. Although abnormalities in other brain regions, including the anterior cingulate, were not evident using voxel-based morphometry, we nonetheless suggest they play an important role in the pathogenesis of OCD consistent with our prior work
(7,
8) . Abnormalities in other brain regions, including the caudate nucleus, globus pallidus, and thalamus, were not evident at the specified significance threshold using voxel-based morphometry, but differences between this approach and regional volumetry preclude firm conclusions regarding their role in the pathophysiology of OCD.