Anorexia nervosa is a serious psychosomatic illness with high morbidity and significant lifetime mortality. About half of patients with anorexia nervosa show a chronic course of the disease despite treatment
(1) . Clinical characteristics seen in anorexia nervosa patients are maladaptive preoccupations with food, weight, and body shape that manifest in stereotyped and rigid behaviors controlling eating and weight. These characteristics are seen as symptomatic expressions of obsessive-compulsive temperament traits, which frequently precede the onset of the disorder
(2) and are associated with a negative course of the disease
(1) . Consistent with an obsessive-compulsive phenotype, anorexia nervosa patients show high concern over mistakes and a relentless perfectionism
(3) .
On a neuropsychological level, these clinical traits of cognitive-behavioral inflexibility in anorexia nervosa can be conceptualized as impaired cognitive set shifting (i.e., concrete and rigid approaches to changing rules) and impaired behavioral response shifting (i.e., stereotyped or perseverative behaviors). The construct of set shifting in anorexia nervosa has been used as a broad and underspecified term without a clear differentiation of cognitive set shifting from behavioral response shifting. A meta-analysis of neuropsychological studies
(4) found a consistent deficit in set-shifting ability in anorexia nervosa; deficits were found in the ill and recovered stage of the disease and also in discordant sister pairs, which suggests that impaired set shifting is a characteristic trait in anorexia nervosa
(4,
5) .
Numerous studies have shown that fronto-striato-thalamic pathways play a pivotal role in shifts of cognitive set and behavioral response
(6 –
8) . In functional neuroimaging studies, the lateral prefrontal cortex and the anterior cingulate/ventromedial prefrontal cortex have been regularly found to be related to cognitive set shifting and inhibitory control
(7 –
9) . Subcortical regions such as the thalamus, the subthalamic nucleus, and the striatum facilitate shifts in behavioral response mediated by the prefrontal cortex
(10,
11) .
Brain imaging studies addressing frontal-subcortical circuits in the resting state in anorexia nervosa have shown increased metabolic rates in the subcortical striatum, thalamus, and brainstem and prefrontal alterations in the medial prefrontal and anterior cingulate cortex
(12 –
15) . There is preliminary evidence for altered dopamine signaling in the striatum that is known to be involved in executive control, habit learning, and reward pathways
(16,
17) .
Functional MRI (fMRI) studies of anorexia nervosa have been primarily concerned with the functional neuroanatomy of the processing of eating disorder-related and reward-relevant cues
(18) . In this study, we used an established visual flexibility paradigm with non-affect-laden stimuli (geometric figures) that allows for a differentiation between shifts in behavioral response with and without shifts in cognitive set
(8) . Behavioral response shifting required study subjects to inhibit a prepotent impulse in favor of an alternative, less automatic behavior. Cognitive set shifting required the subject to change stimulus-response associations.
We hypothesized, first, that anorexia nervosa patients would show impaired behavioral response shifting and, second, that behavioral findings would be more profound in anorexia nervosa in behavioral-shift trials when additional shifts in cognitive set are required. In addition, we predicted that these impairments in executive control in anorexia nervosa would be associated with altered activations in fronto-striato-thalamic loops.
Method
Participants
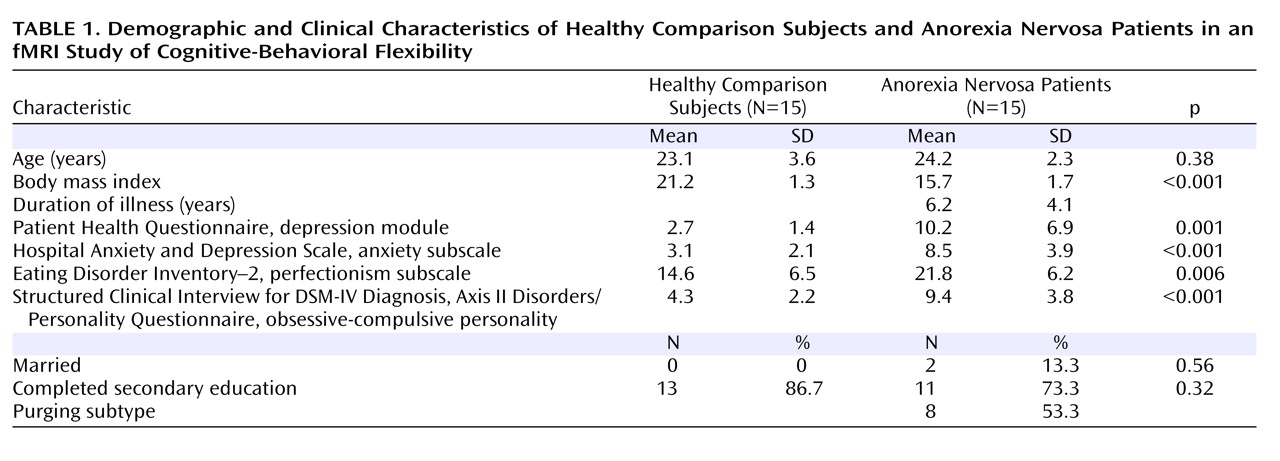
A total of 30 women were enrolled: 15 anorexia nervosa patients (seven with the restricting subtype) and 15 age-matched healthy women in the normal weight range and with no lifetime diagnoses of psychiatric illness. Diagnosis was made using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; 19). Inclusion criteria were age between 18 and 30 years and right-handedness. Exclusion criteria were metallic implants, claustrophobia, current psychopharmacological medication, life-threatening condition, poor German-speaking ability, and lifetime diagnosis of obsessive-compulsive disorder, major depressive disorder, bipolar disorder, psychosis, and alcohol or drug abuse or dependence. Patients were recruited from our wards and outpatient clinic, and comparison subjects were recruited through public advertisements. Written informed consent was obtained from all participants, as approved by the ethics committee of the University of Heidelberg Medical School.
Procedure
Before entering the study, participants were interviewed with the SCID to screen for lifetime psychiatric diseases
(19) . They also completed a battery of self-report questionnaires, including the Eating Disorder Inventory–2
(20), the nine-item depression module of the Patient Health Questionnaire
(21), the anxiety subscale of the Hospital Anxiety and Depression Scale
(22), and the Structured Clinical Interview for DSM-IV Personality Disorders
(19) for an obsessive-compulsive personality disorder. Participants were asked to avoid eating and drinking caffeine-containing beverages for 1 hour and alcohol for 24 hours before the experiment.
fMRI Task
We used a visual target-detection task in an event-related design
(8) . The stimuli were geometric shapes (squares, triangles, and circles) of different colors and sizes. Participants were required to classify each stimulus on the basis of its shape as target, non-target, or standard stimuli. They were required to respond to non-target and standard stimuli with one button press and to target stimuli with an alternative button press.
The stimuli were centered on a white background for 500 msec, with a stimulus interval of 1000 msec. In each of the 11 runs, infrequently occurring target (five per run) and non-target (five per run) stimuli were embedded in frequently occurring standard (154 per run) stimuli. The infrequent target and non-target stimuli were separated by a minimum of nine standard stimuli to allow adequate spacing between events to prevent an overlap of event-related hemodynamic responses.
Before each run, the target stimulus was defined as either a circle (C) or a triangle (T). The target changed after every two runs (TTCCTTCC), resulting in conditions where the target either changed at the start of the run (TC or CT: “shift” runs) or stayed the same from the previous run (TT, CC: “maintain” runs). Targets occurring during “maintain” runs required behavioral shifts only, that is, changing which response button to press. In contrast, those occurring during “shift” runs required shifts in behavioral response and stimulus-response associations (cognitive set shifting), that is, the target had changed from the previous run.
Immediately before the scanning session, participants were trained on the task using a personal computer. In the scanner, participants viewed the stimuli on a semitransparent screen via a head-coil-mounted mirror system. Accuracy (percent correct) and reaction time data were acquired for each trial.
fMRI Acquisition
Images were acquired with a Siemens Trio 3-T scanner equipped with a standard single-channel head coil. We used a rapid echo-planar imaging sequence covering the whole brain with the following parameters: repetition time=2 seconds, echo time=30 msec, flip angle=80 degrees, 33 slices parallel to the anterior commissure-posterior commissure line, slice thickness=4 mm, in-plane resolution 3.4×3.4 mm. In addition, a high-resolution sagittal T 1 -weighted image was acquired with the following parameters: 176 slices, voxel size 1×1×1 mm, repetition time=11 msec, echo time=4.92 msec, flip angle=15 degrees.
Data Analysis
Data analysis was performed with SPM2 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London) implemented in MATLAB, version 7 (MathWorks, Natick, Mass.). The images of the first run were discarded (setup of the first “maintain” run), and the analyses were based on the images of the five “maintain” and five “shift” runs that followed. Functional images were corrected for differences in slice acquisition timing and were realigned with the allowed motion limited to ±3 mm translation and to ±3 degree rotation. Coregistration was performed with the high-resolution T 1 -weighted image. Both functional and structural images were normalized to the Montreal Neurological Institute template. Functional images were spatially smoothed with a kernel of 8 mm full width at half maximum. Low-frequency signal drifts were removed using a 128-second high-pass filter, and temporal autocorrelation in the fMRI time series was corrected using a first-order autoregressive model.
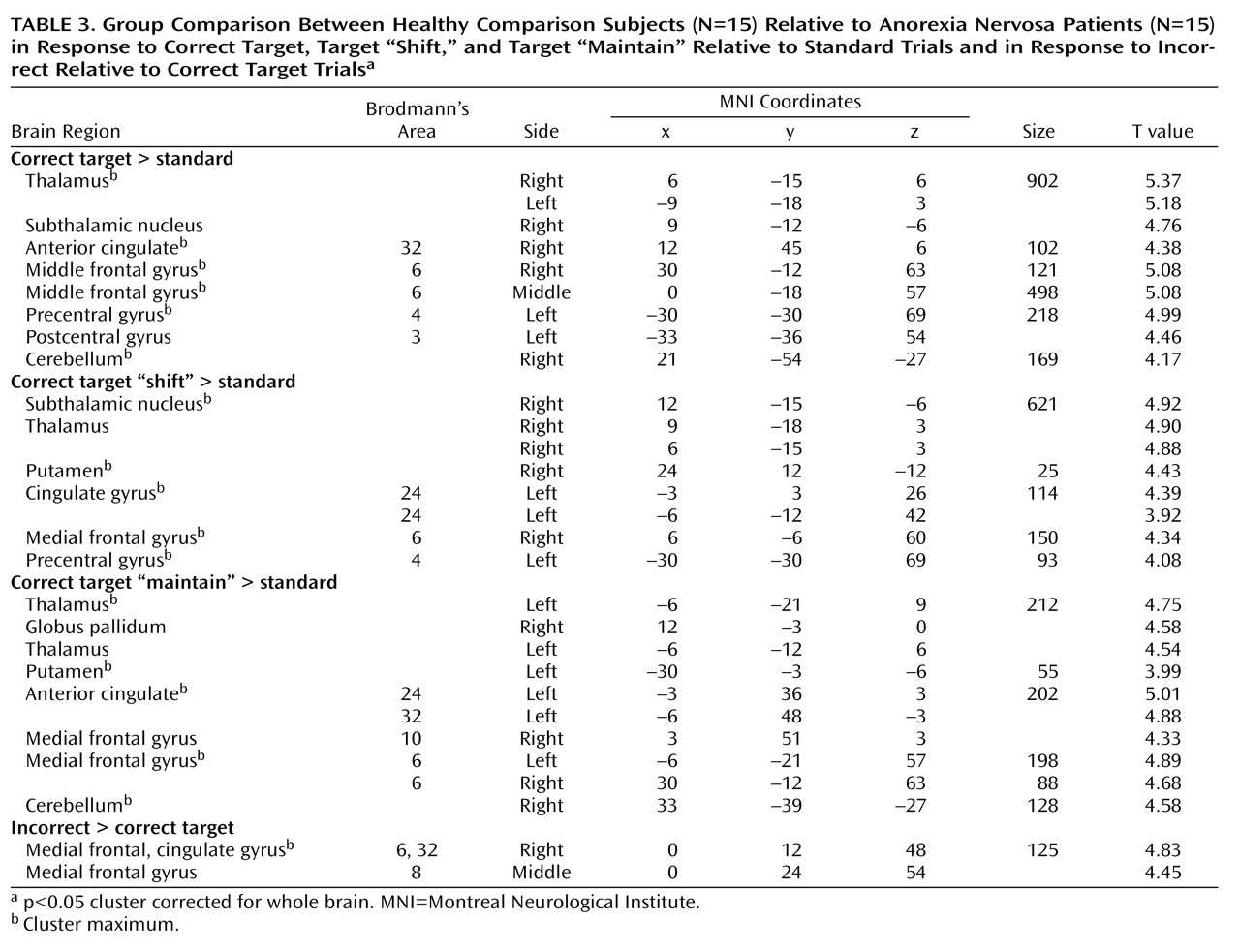
A general linear model was fitted to the single-subject data using six regressors of interest for each subject (target “shift,” target “maintain,” non-target “shift,” non-target “maintain,” standard “maintain,” and standard “shift”), which were modeled as stick functions convolved with the canonical hemodynamic response function. Only correct trials were considered for analyses, except for target trials. T contrasts were calculated for the following comparisons: 1) correct target relative to standard stimuli; 2) correct target “shift” relative to standard stimuli; 3) correct target “maintain” relative to standard stimuli; 4) correct non-target relative to standard stimuli; and 5) incorrect relative to correct target stimuli.
For the group analysis, single-subject contrast images were entered into a hierarchical model equivalent to a random-effects model, allowing population inference
(23) . Generic group maps for anorexia nervosa patients and comparison subjects and between-group comparisons were computed with one-sample t tests and two-sample t tests, respectively. Correlations of depression and anxiety with brain activation were determined by entering scores from the Patient Health Questionnaire or the anxiety subscale of the Hospital Anxiety and Depression Scale and contrast images (correct target relative to standard stimuli) into a simple regression model. All results reported are significant at a cluster-level-corrected threshold of p<0.05.
The behavioral data were analyzed with repeated-measures analyses of variance to determine within-subject effects and group-by-stimulus interaction. Between-group differences were analyzed with Student’s t tests or chi-square tests as appropriate. A p value <0.05 (two-tailed) was considered statistically significant.
Discussion
This is the first study to show that anorexia nervosa patients have altered activation in fronto-striato-thalamic pathways during the performance of behavioral response shifting. These alterations were specific to shifts in behavioral response: except for thalamic activation, they were not observed in response to non-target trials that required recognition of the stimulus but no alteration in behavioral response. In the main analysis we focused on correct trials, but we also addressed error-related brain activation to investigate control processes. Anorexia nervosa patients showed less error-related activation than did comparison subjects in prefrontal regions involved in conflict monitoring and error detection. These findings are in accord with a previous EEG study that reported decreased error monitoring (i.e., error-related negativity) in anorexia nervosa
(24) .
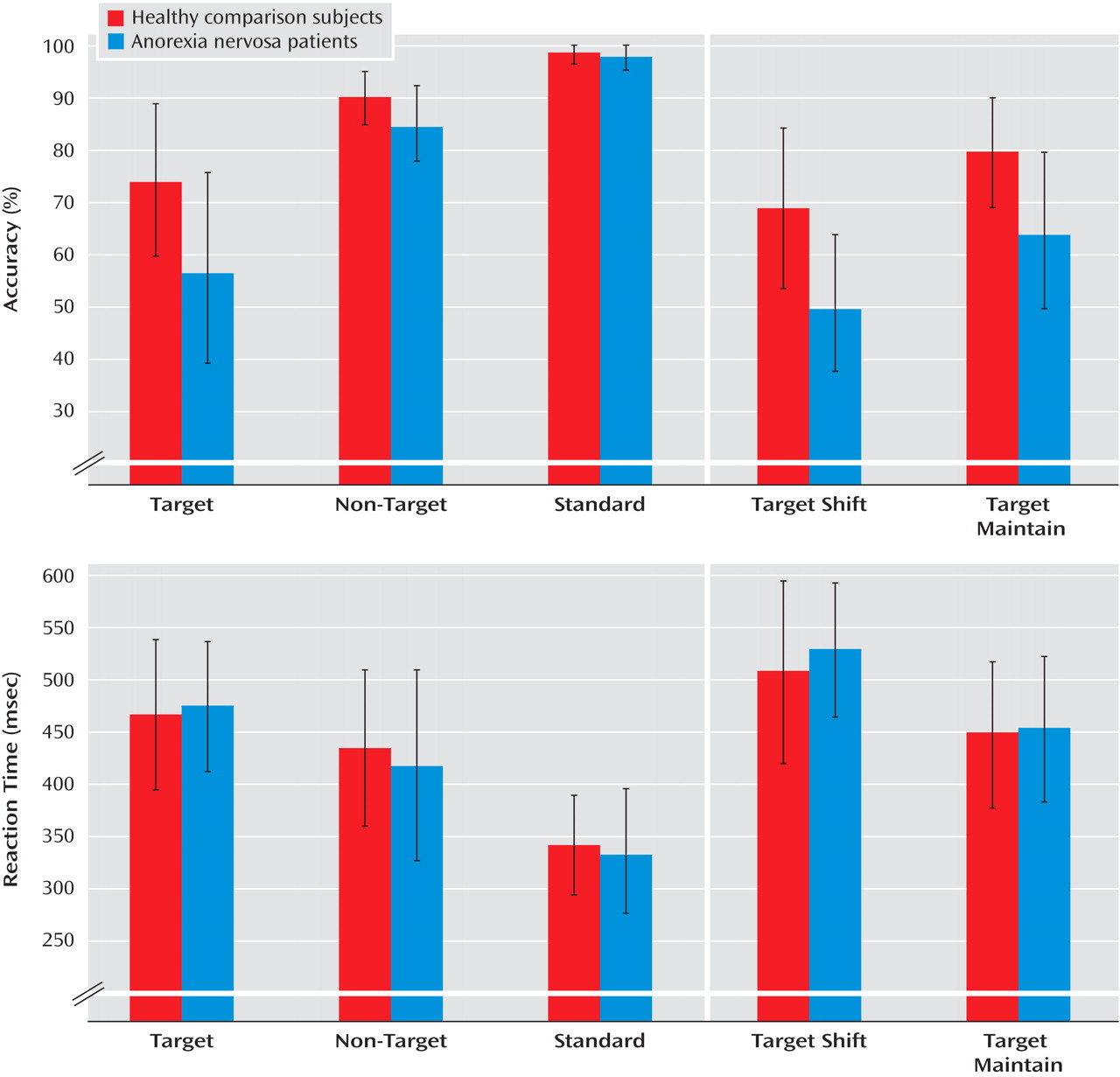
The executive function paradigm differentiated the anorexia nervosa patients from the comparison group in that anorexia nervosa patients were less accurate (i.e., they made more commission errors) in behavioral response shifting. Commission errors in the patient group were not due to greater impulsivity or careless responses (i.e., shorter reaction times) but rather resulted from increased behavioral rigidity. Behavioral adjustments following an erroneous response did not differ between patients and comparison subjects.
Contrary to our second hypothesis, the deficit in executive control was independent of whether behavioral “shift” trials involved additional shifts in cognitive set; patients and comparison subjects showed a similarly increased switching cost (longer reaction times, higher error rates) to additional cognitive set shifting. Previous evidence suggests that anorexia nervosa patients might be especially prone to impaired cognitive set shifting, although none of the published studies used selected tasks designed to differentiate specific processes of cognitive-behavioral inflexibility
(4) . For example, the Wisconsin Card Sorting Test, one of the most frequently used tasks, has a complex design that includes additional cognitive processes, such as implicit learning (rules are covertly changed). Our data in this study indicate that anorexia nervosa patients demonstrate no deficit in cognitive set shifting when the change of the stimulus-response association is overtly displayed. Instead, clinical manifestations of stereotyped and rigid behaviors in anorexia nervosa may reflect poor generation of shifts in behavioral response. This finding is supported by impaired behavioral response shifting in neuromotor function tests, such as diadochokinesis and two-finger tapping
(25,
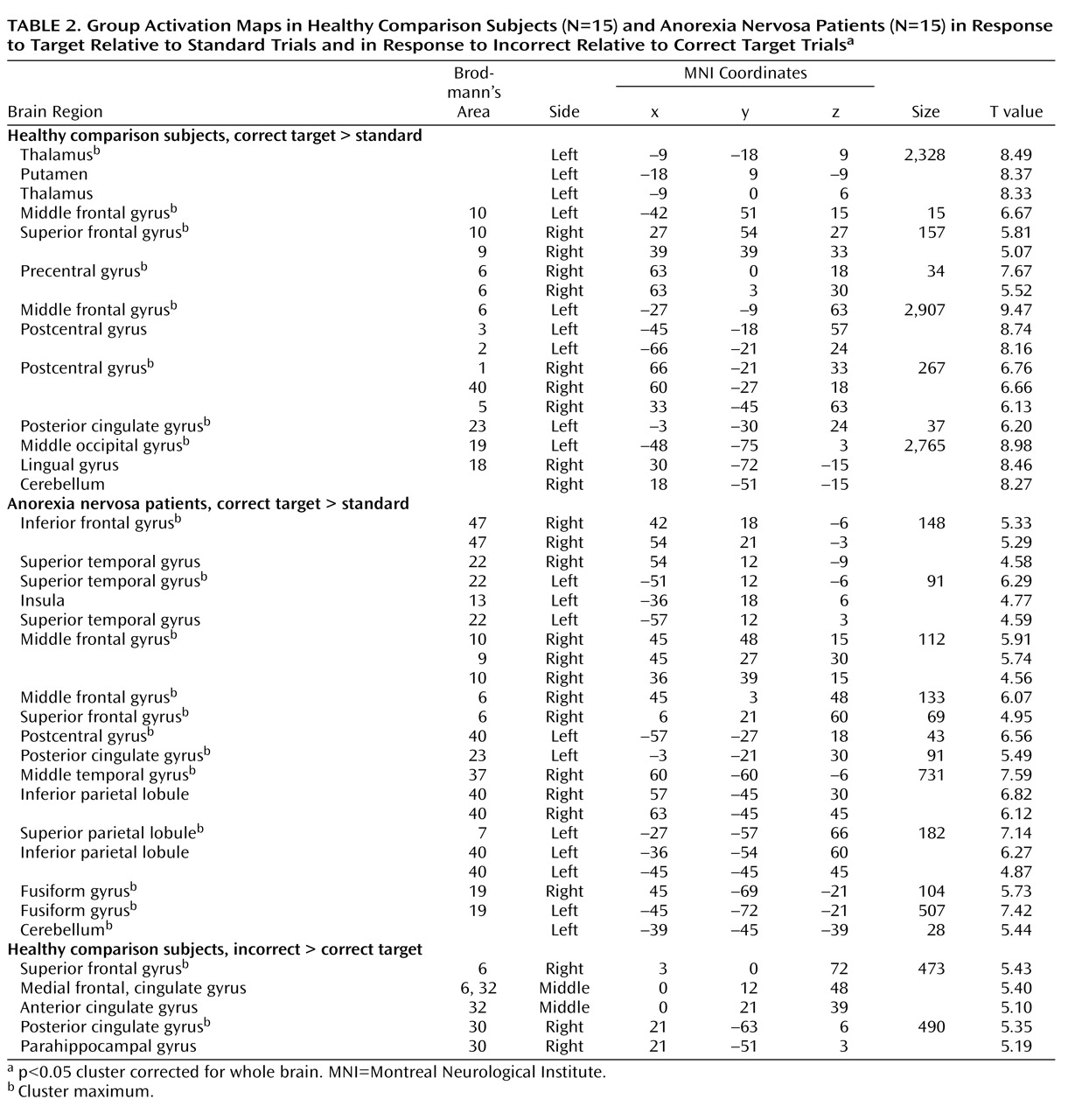
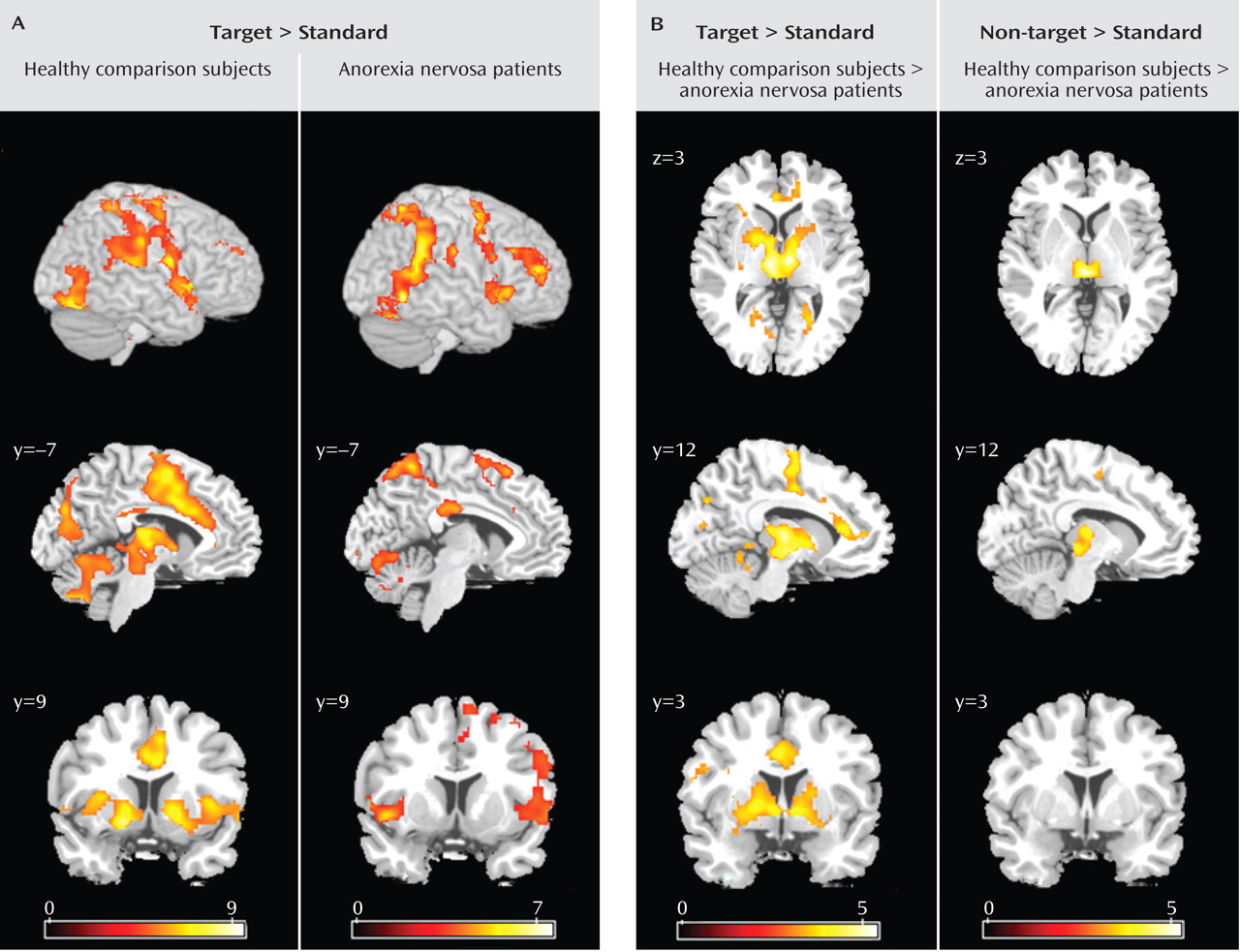
26) . Further studies that systematically differentiate between shifts in behavioral response and cognitive set in anorexia nervosa are needed to corroborate our findings. The comparison group activated the established neural network involved in shifts of behavioral response and cognitive set, including subcortical brain regions (the thalamus and striatum), the dorsolateral prefrontal cortex, the anterior insula/ventrolateral prefrontal cortex, the parietal cortex, premotor brain regions reaching into the dorsal anterior cingulate cortex, and the cerebellum
(7,
8) . In contrast, anorexia nervosa patients showed a failure to recruit subcortical (thalamus, striatum) and frontal (anterior cingulate cortex, supplementary motor area) brain regions, which differed significantly from the comparison group. Patients showed preserved neural activation in the right ventral frontoparietal network (the right middle frontal gyrus and the temporoparietal junction bilaterally) that was not observed in the comparison group. This cortical system is involved in shifting attention and interruption of ongoing cognitive activity, when infrequent behaviorally relevant stimuli occur
(27) . The dominance of higher-order association cortices (the frontoparietal network) over impaired ascending motivational processes has been reported in a study using a gambling task
(17) . These findings suggest that anorexia nervosa patients show a pattern of predominant effortful and supervisory cognitive control in response to diverse tasks. The results were independent of task performance, so these different activation patterns are likely to reflect the use of alternative strategies during behavioral response shifting.
The most prominent finding in patients relative to comparison subjects was decreased bilateral thalamus activation during the processing of infrequent events (target and non-target stimuli). The thalamus is part of an alerting network that allows reflexive distribution of relevant sensory information to numerous divergent brain regions
(28,
29) and also plays a relevant role in higher-order cognition through reciprocal cortico-thalamo-cortical projections
(30,
31) . Notably, the thalamus activation in comparison subjects was greater to behavioral response shifting compared with non-target processing, which supports top-down priming of target selectivity and reactivity within the thalamus. The mediodorsal nuclei of the thalamus were most activated. This area is closely connected to the ventral striatum and prefrontal cortex as part of a neural circuit that facilitates behavioral response shifting mediated by the prefrontal cortex
(6,
11) . The finding of impaired thalamus function in anorexia nervosa patients is supported by brain imaging studies in the resting state
(32,
33) .
Patients also had impaired activation of the anterior cingulate cortex and the putamen during behavioral response shifting. The anterior cingulate cortex subserves performance monitoring and cognitive conflict detection
(34,
35) and has been implicated in motivational and evaluative aspects (i.e., reward, reinforcement learning) to control and initiate behavior
(36,
37) . Therefore, the findings of decreased anterior cingulate cortex and supplementary motor area activation in anorexia nervosa patients may indicate reduced motivation and initiative drive to nonroutine (infrequent) challenges. The impaired activation of the anterior cingulate in anorexia nervosa is supported by previous morphometric and functional brain imaging studies
(12 –
14) .
Relative to comparison subjects, patients showed decreased activation of the ventral striatum (putamen). The ventral striatal circuit receives input from the anterior cingulate cortex, ventromedial prefrontal cortex, hippocampus, and amygdala
(6), forming a pathway involved in motivational processing and the elimination of inappropriate response options
(38,
39) . This is in accord with studies of anorexia nervosa that have reported reduced ventral striatal activation to wins and losses, altered dopamine D
2 /D
3 receptor binding of the ventral striatum, or a generally reduced sensitivity of the appetitive motivational system
(16,
17,
40) . However, the similarities with the gambling task of Wagner et al.
(17) were restricted to the ventral striatum system, as we found no group differences between patients and comparison subjects in dorsal striatum activation.
In summary, we found decreased task-related activation at different levels within the anterior cingulate-striato-thalamic network. This network is involved in motivational processes subserving the initiation of goal-directed behavior. Thus, the neural findings support the thesis that the behavioral impairment reflects poor initiation of behavioral response shifting.
Limitations
Our sample size was insufficient to examine diagnostic differences between subgroups (e.g., restricting versus purging subtypes of anorexia nervosa). Since we investigated exclusively chronically ill patients, we were not able to control for any potential exacerbating effects of prolonged starvation. Additional region-of-interest analyses do not indicate a pattern of a global and nonspecific effect of hypoactivation/reduced blood flow in anorexia nervosa patients. Further studies in patients who have recovered will be important to clarify this issue. Comorbid depression and anxiety may have influenced the findings. However, the levels of depression and anxiety were not correlated with brain activation. Focusing the main outcome on correct trials may have limited our ability to test our second hypothesis, namely, the differentiation of shifts in behavioral response and cognitive set. This was acceptable, however, because on a behavioral level, no group differences between the two conditions were observed.