Given the high rates of nicotine addiction among individuals with psychiatric disorders
(1,
2), readers of the
Journal may be interested to know that the substantial genetic risk
(3) for nicotine addiction is being elucidated, as exemplified by an article in this issue by Bierut et al.
(4) . In the past year, several studies have identified a cluster of nicotinic receptor subunit genes, located on chromosome 15q25, as conveying risk for nicotine addiction and other disorders.
Nicotine acts as an agonist at brain nicotinic receptors, for which the endogenous neurotransmitter is acetylcholine. Each nicotinic receptor consists of five subunits, which form a central cation (Ca
++, K
+, Na
+ ) channel. The great heterogeneity of nicotinic receptors derives mostly from the multiple subunits (each with its own gene), including α2– α10 and β2– β4, which combine promiscuously to yield a wide array of brain nicotinic receptors. The specific composition of the five subunits in the receptor conveys important pharmacological characteristics of the receptor and its cation channel
(5) .
The complex history of recent genetic reports on these receptors and smoking begins with a genome-wide association study of about 1,000 individuals with nicotine addiction and about 900 comparison subjects
(6) . The study examined DNA variation across the genome, reporting limited statistical evidence for association with alleles in a cluster of nicotinic receptor genes— α3 (CHRNA3), α5 (CHRNA5), and β4 (CHRNB4) on chromosome 15q25. This report was followed by several other genome-wide association studies, implicating the same alleles, with much stronger statistical significance (see
Table 1 ). The increased risk conveyed by the risk alleles is modest, with an odds ratio of ∼1.3.
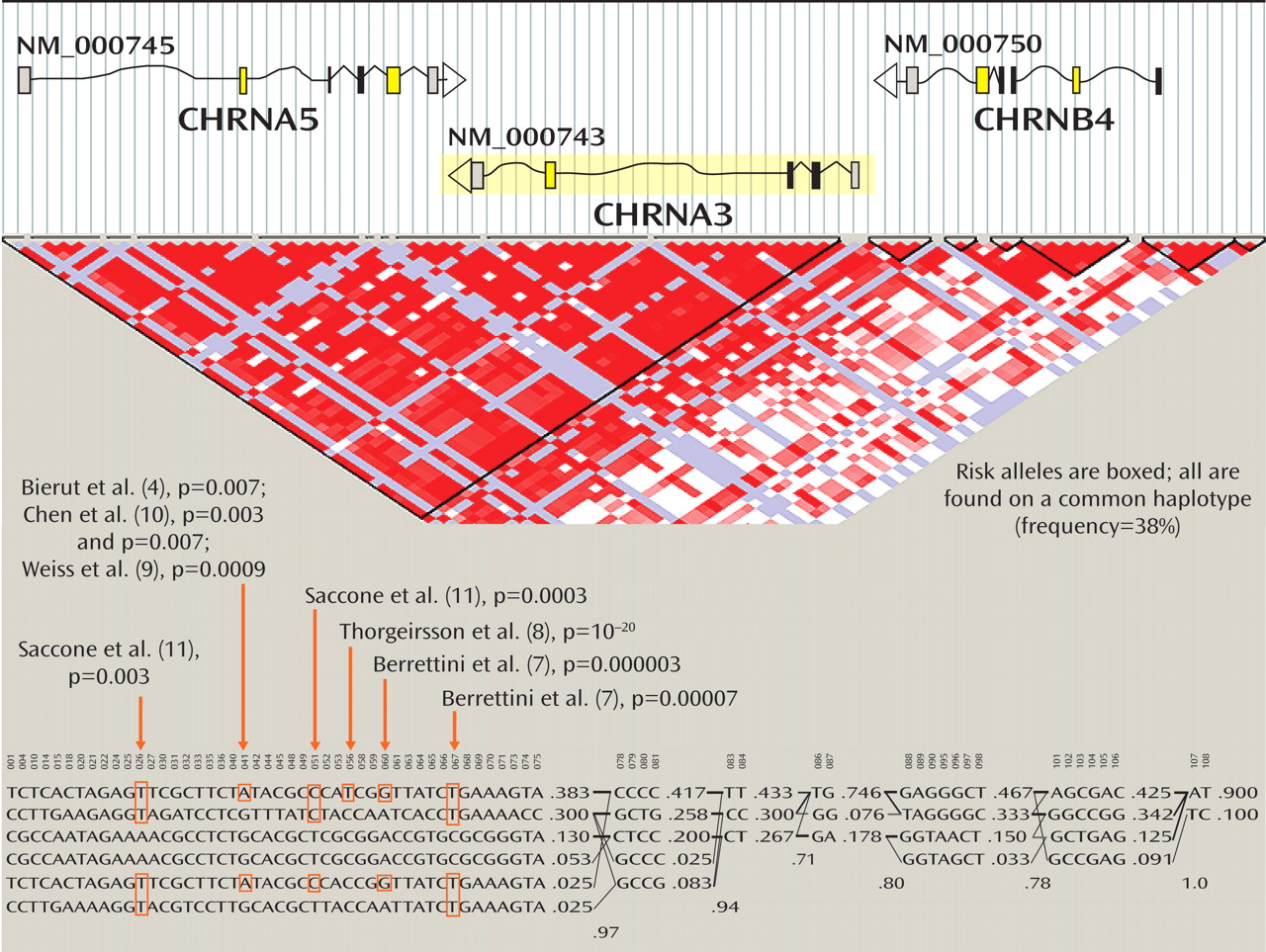
The top part of
Figure 1 presents diagrammatic sketches of the three nicotinic receptor subunit genes in the implicated region; the boxes indicate exons and the arrows indicate the direction of transcription. CHRNA3 is shown in yellow because it is coded in the reverse direction from CHRNA5 and CHRNB4. In the triangular diagram below the gene diagrams, the red areas indicate regions of high correlation among alleles (that is, the likelihood that alleles are found together, known as linkage disequilibrium), and white and blue colors indicate regions of less linkage disequilibrium. Some combinations of these alleles occur very frequently in the same individuals and form a haplotype. Haplotypes are combinations of several polymorphisms that are often inherited intact across generations and in populations with a common ancestry, such as European Americans. Alleles and their combination in haplotypes in this CHRNA3-CHRNA5-CHRNB4 cluster were thus identified as conveying risk for nicotine addiction.
The most common European haplotype in the region is the first one listed in the figure, which has a 38% frequency. This common haplotype increases risk for nicotine addiction, and it has the risk alleles identified in these studies. The second most common haplotype contains some but not all of the risk alleles. Next, there are two less common European haplotypes that are protective, containing none of the risk alleles (the third and fourth haplotypes, with 13% and 5.3% frequencies in Europeans). The last two haplotypes are uncommon among European-origin individuals, and contain some of the risk alleles.
Within this nicotinic receptor gene cluster, there may be more than one risk allele that alters gene function
(4) . The article by Bierut et al. in this issue of the
Journal (4) reports that a nicotine addiction risk variant in the a5 nicotinic receptor gene produces a receptor with distinct binding constants and unique electrophysiologic properties. The effect of the variant at the level of the receptor’s neurobiological function thus provides a possible clue to which alterations in brain biology increase the risk for nicotine addiction.
To complicate matters further, two reports from large-scale genome-wide association studies
(12,
13) have concluded that these same alleles increase the risk for lung cancer in people of European origin, with an odds ratio of 1.3. The increase in risk for lung cancer attributable to these sequences may be an example of how a genetic variant leads to a change in brain biology and thus to a change in behavior, in this case nicotine addiction, that ultimately causes cancer through habitual smoking. However, the lung cancer association may also be independent of the risk for nicotine addiction and may involve nicotine’s ability to inhibit apoptosis (programmed cell death;
14 ), promote cell proliferation
(15), and protect against cytotoxic causes of cell death
(16), making survival of tumor cells more probable.
Clearly, additional research is needed to identify more definitively all the functional variation in this complex region. Some of this variation will likely be detected in other ethnic groups, where variants in these genes may also occur. However, it is reasonably clear that at least one common haplotype increases risk for nicotine addiction. This information can be used to initiate research projects of potentially important public health impact, including the following:
1. Develop new nicotine addiction medications that “normalize” nicotinic receptor function, differences attributable to the nicotine addiction risk alleles, versus the “protective” alleles. Presumably such medications would be more efficacious in persons with the nicotine addiction risk alleles at this locus.
2. Test existing nicotine addiction pharmacotherapies to determine whether the nicotine addiction risk alleles predict response to bupropion, varenicline, or nicotine replacement therapies.
3. Create new prevention programs designed for adolescents possessing the nicotine addiction risk alleles, a laudable goal that would require genotyping a large target population and prospective assessments over years. This opportunity may be especially relevant given evidence that these alleles may preferentially predispose individuals to early onset of smoking
(9,
17) .