Findings on the relationship of minor physical anomalies to SSRI exposure have been inconsistent. The occurrence of three or more minor anomalies is important because of its predictive association with major structural malformations
(11), neurodevelopmental abnormalities, or eventual psychiatric problems
(12) . Although a higher risk for three or more minor anomalies in fluoxetine-exposed infants was reported
(6), no increased risk was found in newborns exposed to sertraline
(13) or SSRIs generally
(14), compared to unexposed newborns.
The landmark 1996 publication by Chambers et al.
(6) shifted investigative attention beyond first-trimester SSRI treatment and malformations to later exposure and other reproductive outcomes. Significantly lower maternal weight gain and infant birth weight were reported after exposure to fluoxetine after 25 weeks’ gestation
(6) . Subsequent investigators reported that compared to unexposed infants, newborns exposed to SSRIs had a mean birth weight 188 g lower
(14) and a significantly higher proportion below the 10th percentile in weight
(15) . Birth weights in SSRI-exposed neonates also have been comparable to those of unexposed infants in several investigations
(4,
5,
16) ; similarly, maternal weight gain also was equivalent
(4,
5) .
Neonates exposed in late pregnancy have a threefold higher risk for neonatal syndrome (CNS, motor, and respiratory signs), which resolves within 2 weeks after birth
(22) . Chambers et al. reported that nearly one-third (31.5%) of neonates exposed to fluoxetine in the final trimester had poor neonatal adaptation, compared to 8.9% of neonates with earlier exposure only
(6) . Several investigators
(14,
16,
23,
24) have reported less favorable Apgar scores associated with third-trimester SSRI exposure. A specific association between late-pregnancy fluoxetine exposure and persistent pulmonary hypertension of the newborn has been reported
(25) . Two (2.7%) of 73 exposed infants developed persistent pulmonary hypertension of the newborn, a rate six times that in the general population (0.1–0.2%). Additional reports of neonatal respiratory distress after SSRI exposure have been published
(15,
24,
26) .
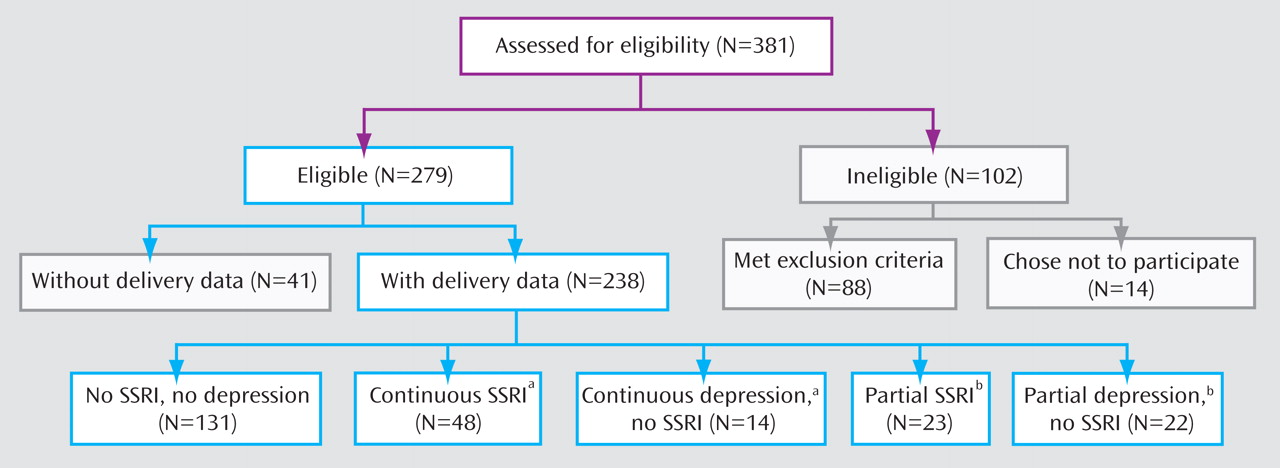
We conducted a prospective observational study to determine whether either SSRI treatment or untreated depression in pregnant women with major depressive disorder, compared to unexposed pregnant women without major depression, was associated with increased risk for 1) minor physical anomalies, 2) reduced maternal weight gain and infant birth weight, 3) preterm birth, or 4) poor neonatal outcomes. To our knowledge, this is the only prospectively followed study group with subgroups for both continuous and partial exposures to depression and SSRIs.
Method
In this prospective observational study, maternal assessments were completed at weeks 20, 30, and 36 weeks of gestation. Delivery records were reviewed and the newborns examined at 2 weeks postpartum.
Study Subjects
The pregnant women were 15–44 years old and were recruited from two sites. Twenty-one were enrolled in Cleveland between Jan. 23, 2000, and April 1, 2001, and 217 were recruited in Pittsburgh between April 23, 2003, and July 11, 2007. Recruitment was by self-referral, physician referral, advertising, and screening in obstetrical ultrasound suites. Approval was obtained from the Case Western Reserve University and University of Pittsburgh institutional review boards. All women provided written informed consent.
After evaluating the patterns of SSRI and depression exposure that occurred in our subjects, we created five nonoverlapping groups:
1. No SSRI, no depression (N=131)—no exposure to any antidepressant or to major depressive disorder.
2. Continuous SSRI exposure (N=48)—treatment with an SSRI during the entirety of pregnancy or for the majority of each of the three trimesters.
3. Continuous depression, no SSRI (N=14)—the presence of major depression throughout pregnancy or for the majority of each of the three trimesters, without SSRI treatment.
4. Partial SSRI exposure (N=23)—treatment with an SSRI at some point during pregnancy but at least one full trimester without exposure; this group was equally split between women treated with an SSRI in the first and/or second trimester, but not the third, and women treated in the second and/or third trimester, but not the first.
5. Partial depression, no SSRI (N=22)—major depressive disorder at some point during pregnancy but no depression for at least one trimester, without SSRI treatment.
Consultation about depression management during pregnancy was provided to women with depression or SSRI exposure, and a summary was sent both to the woman and to her physician(s). Enrollment did not depend on acceptance of the recommendations or treatment choice. No intervention was prescribed by the study team.
Exposures
Beginning with conception, SSRI exposure was documented by charting each subject’s drug doses across each week of gestation. Because we observed that nearly 15% of the drug-treated childbearing women in a previous randomized SSRI trial had negligible serum levels
(27), we confirmed exposure (maternal serum level ≥10 ng/ml) for inclusion in the SSRI-treated groups. Women with active substance use disorder (identified by self-report or urine drug screen) or with gestational exposure to benzodiazepines or prescription drugs in the FDA-defined category of D or X were excluded. Exposures to prescribed drugs, over-the-counter medications, environmental agents, alcohol, and smoking were recorded at each assessment.
The diagnosis of major depressive disorder was made according to the Structured Clinical Interview for DSM-IV (SCID). We adapted the timeline technique
(28) to chart depression course by month across the pregnancy. Women with psychosis or bipolar disorder were excluded. Those with multiple gestations or chronic diseases were also excluded.
Outcomes
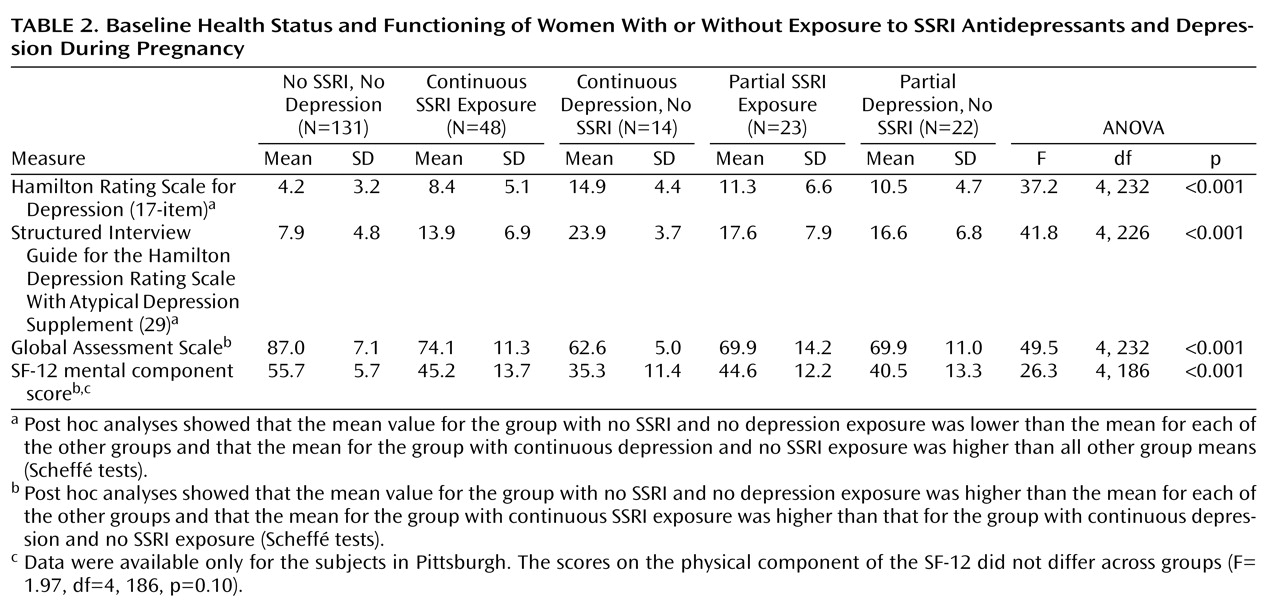
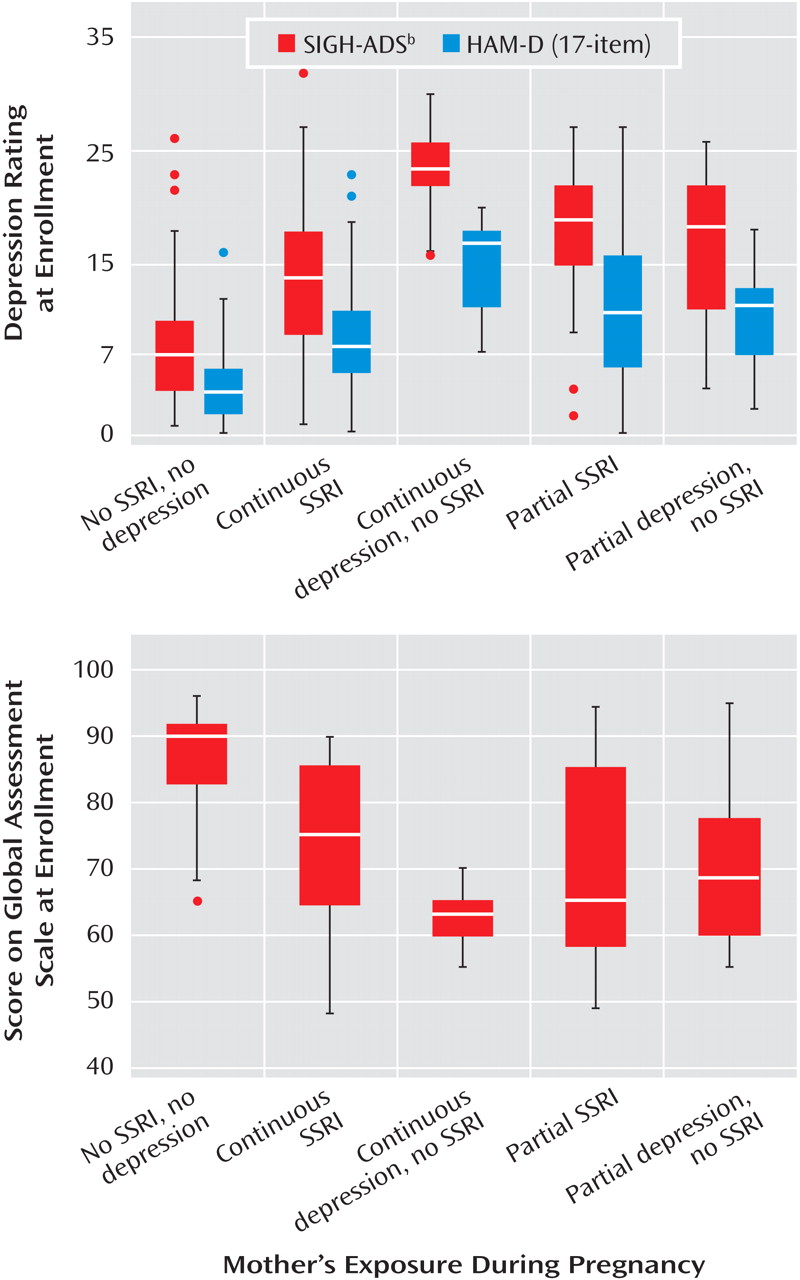
Descriptive data for the sample included demographic characteristics, smoking status, alcohol intake, parity, and previous preterm births. Depression severity was assessed with the 29-item Structured Interview Guide for the Hamilton Depression Rating Scale With Atypical Depression Supplement (SIGH-ADS)
(29), which includes all versions of the Hamilton Rating Scale for Depression. The functional measures were the Global Assessment Scale from the SCID and the SF-12 Health Survey
(30), which yields summary scores for the physical and mental components.
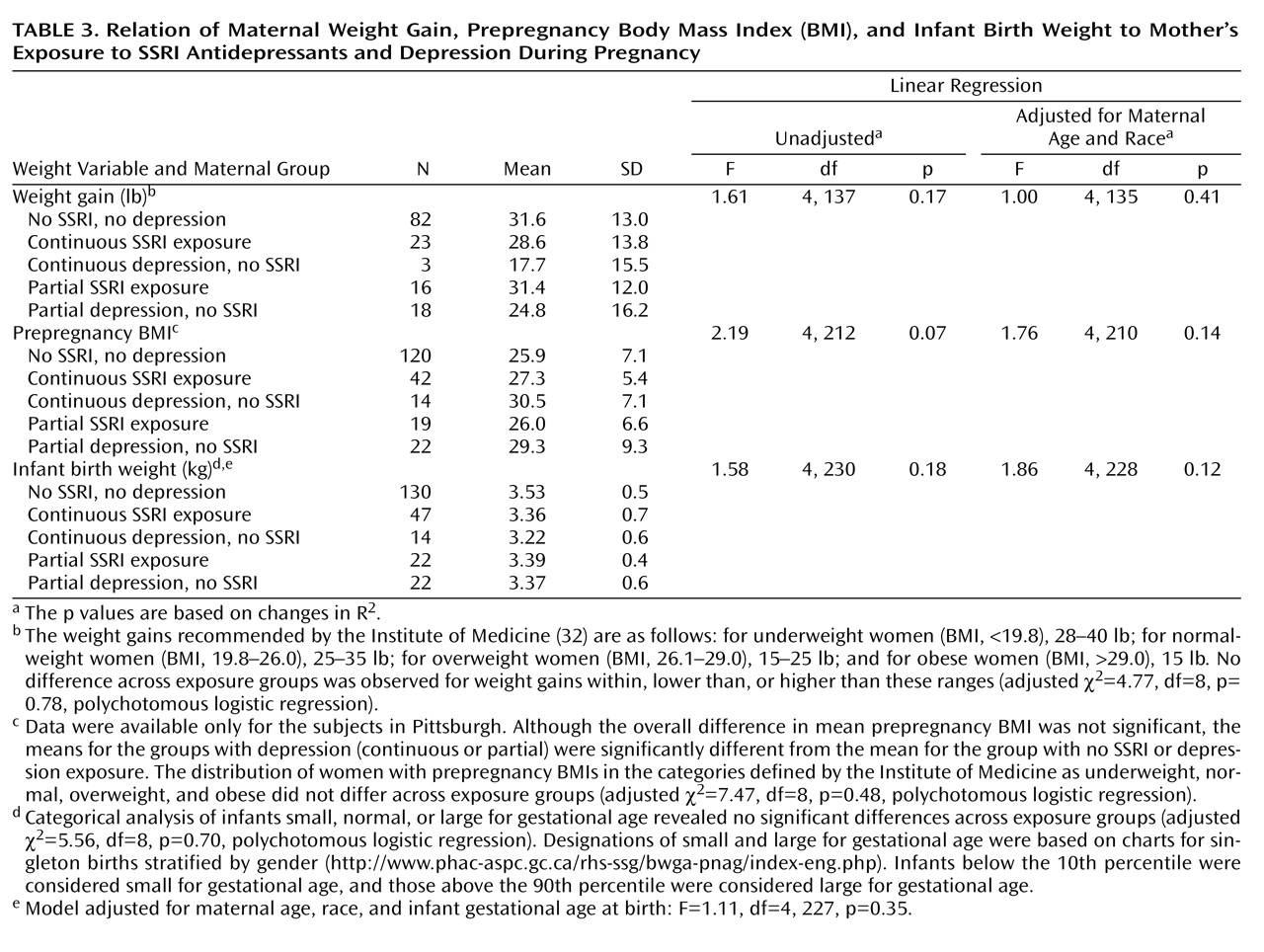
The main outcome measures were minor physical anomalies, maternal weight gain, infant birth weight, pregnancy duration, and neonatal characteristics. To assess minor anomalies, an infant examination was performed at 2 weeks postbirth by a physician (D.L.B.) or pediatric nurse practitioner blind to maternal exposures. The minor anomalies measure was initially developed at the National Institute of Mental Health
(12) to evaluate dysmorphology in children. Minor anomalies specific to teratogen exposure were added (by K.L.W.) specifically for use in drug-exposed pregnant women. The expanded measure was successfully used to compare minor anomalies in infants exposed prenatally to alcohol with unexposed comparison infants
(31) .
Body mass index (BMI) was calculated from the mother’s prepregnancy weight and height. Maternal gestational weight was recorded at each evaluation. The Institute of Medicine guidelines
(32) were used to define categories of both prepregnancy BMI status and adequacy of pregnancy weight gain. Infant birth weight, length, and head circumference and other outcome data were obtained by review of delivery records by independent evaluators (D.F.H. and S.J.-I.) blind to study hypotheses and design. Designations of small (<10th percentile) and large (>90th percentile) for gestational age were standardized by sex.
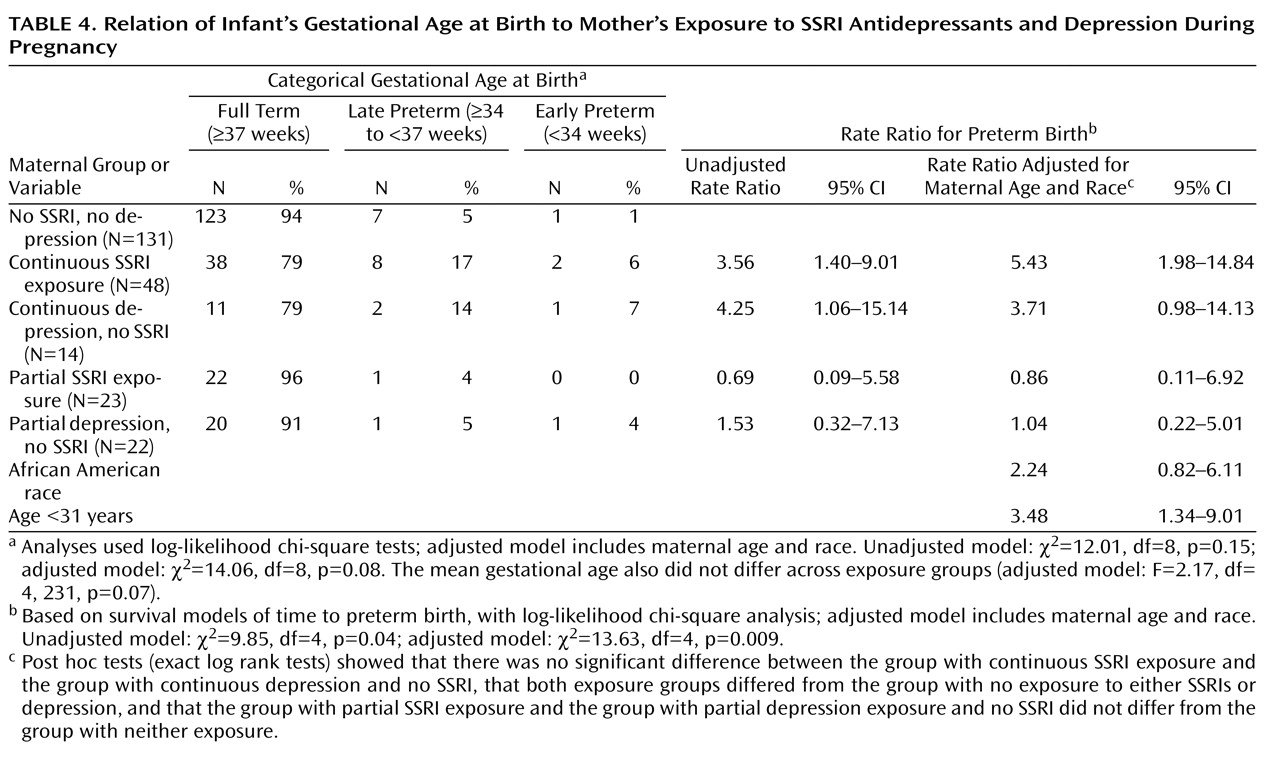
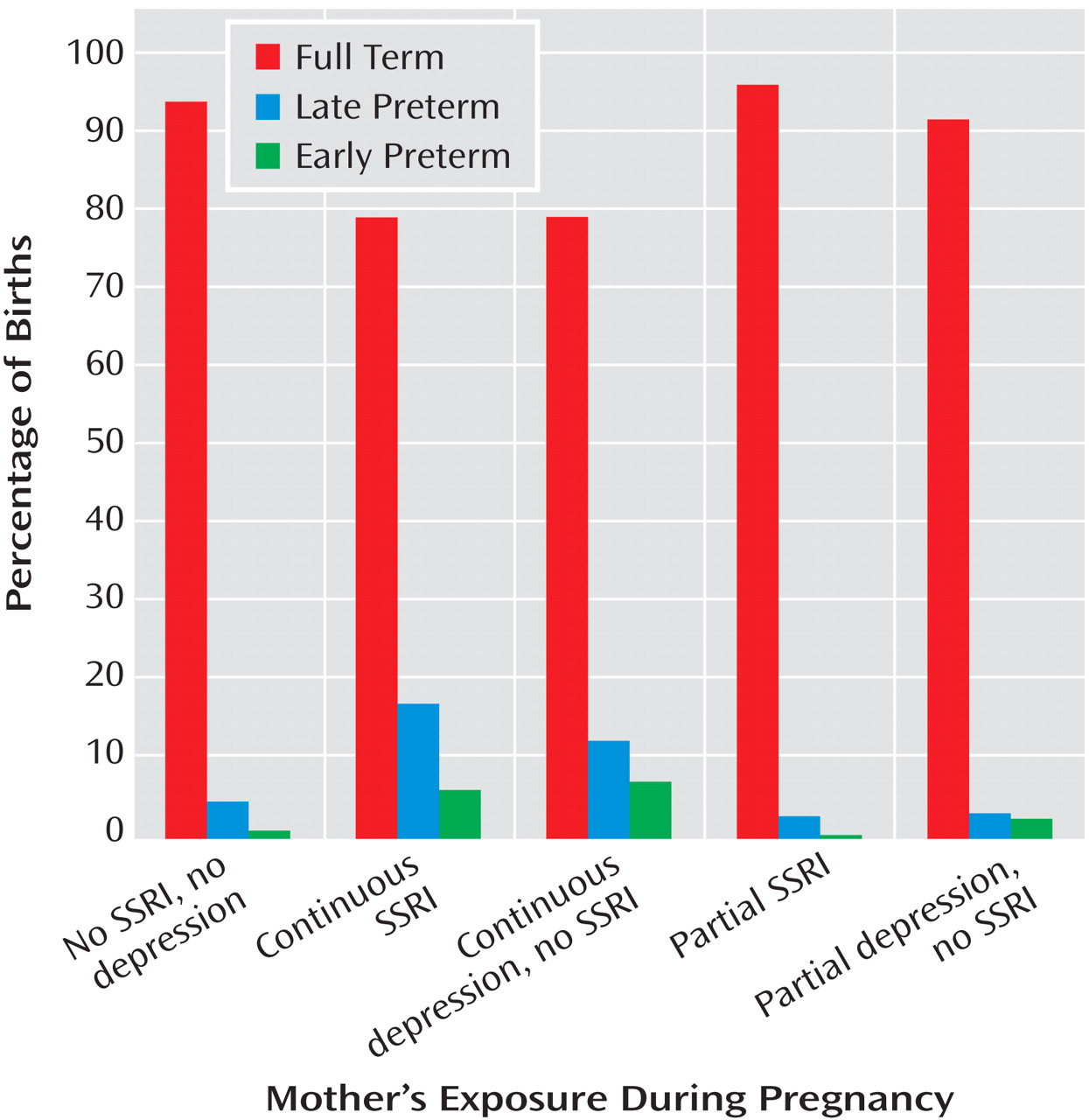
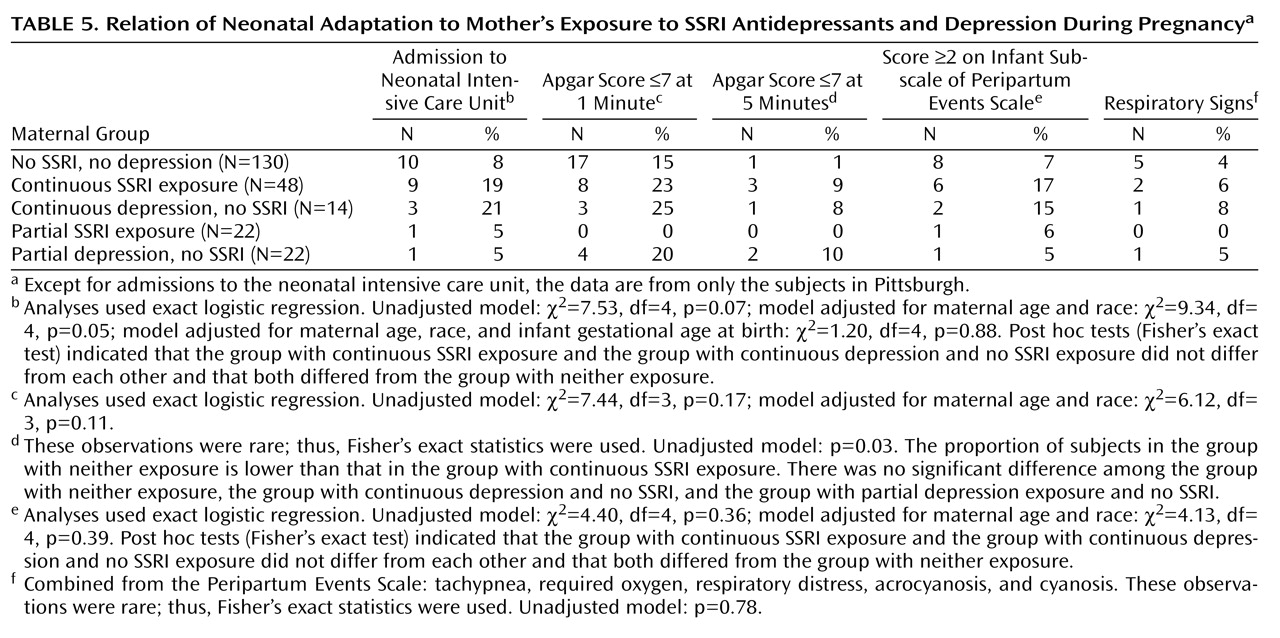
Preterm birth was subcategorized as late (34 to <37 weeks) and early (<34 weeks). Measures to assess neonatal adaptation included delivery method, Apgar scores at 1 and 5 minutes, admission to a neonatal intensive care unit (NICU), and item scores on the infant subscale of the Peripartum Events Scale
(33) . The infant subscale of this measure includes 10 problems (pH correction, volume expansion, transfusion, hypoglycemia, hyperbilirubinemia, hypocalcemia, sepsis, meconium aspiration pneumonitis, other, and other life-threatening). The infant subscale was divided into two categories: one or no problems versus two or more problems; the latter category reflected the most affected 10% of infants. To evaluate respiratory distress, we identified five variables on the Peripartum Events Scale (tachypnea, required oxygen, respiratory distress, acrocyanosis, cyanosis) that were combined and compared across groups.
Statistical Methods
Demographic and historical variables were compared with Pearson chi-square statistics or Fisher exact statistics (for variables with expected cell frequencies of less than 10) for categorical variables and one-way analysis of variance (ANOVA) for continuous measures. Post hoc comparisons were done for variables with significance levels of p<0.05. Outcome measures were compared with regression models. Unadjusted models were performed with the five exposure groups and followed with adjustment for maternal age and race, which were selected because they had prominent effects on reproductive outcomes and were demographic variables that significantly differed among exposure groups.
We analyzed differences in minor physical anomalies 1) among infants exposed to SSRIs, depression, or neither in the first trimester and 2) among the five groups. Minor anomalies were analyzed both as frequency counts with Poisson regression and as a dichotomized variable (fewer than three versus three or more) with logistic regression. Prepregnancy BMI, weight gain at week 36, and infant birth weight were analyzed with linear regression. Categorical prepregnancy BMI and infant birth weight and the proportion of women within Institute of Medicine guidelines for weight gain were analyzed with polychotomous logistic regression.
Gestational age at birth was analyzed both as a continuous measure and as a categorical variable (full term, early preterm, or late preterm). Time to birth across groups was compared with Cox proportional hazard models. The proportions of infants with preterm birth, NICU admission, 1- and 5-minute Apgar scores of 7 or less, Peripartum Events Scale subscale ratings of 2 or higher, and respiratory signs were compared with logistic regression. Adjusted models were not completed for neonatal outcome variables with low frequencies, i.e., fewer than 10 occurrences. Stata version 8.0 (Stata Corp., College Station, Tex.), SPSS version 15.0 (SPSS, Chicago), and StatXact version 8 (Cytel Corp., Cambridge, Mass.) were used.
Discussion
In this controlled prospective investigation, we found that gestational exposure to SSRIs or depression (in unmedicated women) was not related to the number of minor physical anomalies in offspring of women with major depressive disorder. This study and two others
(13,
14) have not replicated the original report
(6) of a higher rate of minor anomalies in infants exposed prenatally to SSRIs. Moreover, no definitively higher risk for two clinical correlates of minor anomalies—major structural malformations
(8 –
10) and neurodevelopmental abnormalities or psychiatric problems
(4,
14,
15,
34,
35) —has been associated with SSRI exposure. However, one investigative team
(36) found normal mental but lower psychomotor skills in toddlers exposed prenatally to SSRIs.
Like other researchers
(5,
7,
18,
37), we found that maternal weight gain was not significantly lower in SSRI-treated women with or without adjustment for preconception BMI. We observed that women with unmedicated depression (continuous or partial) tended to have a pattern of higher mean preconception BMI, coupled with lower mean weight gain, than women treated with SSRIs or comparison subjects.
A major finding of this study is a higher risk for preterm birth in infants exposed in utero to either continuous SSRI treatment for depression or continuous depression without SSRI treatment. Our study joins the converging yet controversial literature that links SSRI treatment to a threefold
(6,
14,
18) increase in the risk for preterm birth. Another important observation is that women exposed to depression (with no SSRI treatment) throughout pregnancy had a comparable level of increased risk for preterm birth.
Attempts to differentiate reproductive outcomes related to SSRIs from those associated with depression have been few
(15,
18) . With a prospective observational design, Suri et al.
(18) studied pregnant women in three groups: 1) antidepressant treatment for more than 50% of the pregnancy, 2) major depressive disorder with no treatment, discontinuation of antidepressant in the first trimester, and/or brief antidepressant exposure, and 3) neither exposure. The rates of preterm birth were 14.3%, 0.0%, and 5.3%, respectively. The authors suggested that SSRI treatment, rather than depression, was associated with the higher risk for preterm birth. We found equivalent effects of continuous SSRI treatment and depression exposure on preterm birth.
One explanation for variable research findings is differing characteristics among the study groups. In this investigation, women who received SSRIs continuously had lower depressive symptom scores and better functioning than the women with continuous depression and no SSRI exposure. In contrast, Suri et al.
(18) studied medicated and unmedicated pregnant women with depression and observed comparable levels of depression. Additionally, the levels of depressive symptoms and functioning in our group with partial SSRI exposure and the group with partial depression and no SSRI exposure were similar, which emphasizes the differential importance of continuous exposure to either SSRIs or depression. For improving risk-benefit decisions about the treatment of major depressive disorder during pregnancy, the crucial question is whether treated women have more favorable reproductive outcomes compared to unmedicated women.
Another explanation for the lack of consistent findings is the variability in exposure definitions across studies. For example, we found that partial exposure to either SSRIs or depression did not increase the risk for preterm birth. Similarly, Chambers et al.
(6) reported that mothers who discontinued SSRIs before the third trimester (similar to our group with partial exposure) had a preterm birth rate comparable to the rate for comparison subjects, while mothers with third-trimester exposure (82% treated throughout pregnancy) had an increased rate. Other investigators have defined exposure as 1) early, with antidepressant use ending before 16 weeks of gestation, without elaboration about later use
(7), 2) late pregnancy
(24), 3) an antidepressant prescription filled during pregnancy
(14,
15), 4) an antidepressant prescription purchased during trimester 1, 2, or 3 or throughout pregnancy
(20), 5) first trimester
(5), and 6) throughout pregnancy or partial treatment
(37) . Standardization of definitions for both SSRI and depression exposures would facilitate cross-study analyses.
In this study group, over 20% of the pregnant women with major depressive disorder who were either continuously treated with an SSRI or continuously exposed to active depression delivered preterm infants. The rates of late- and early-preterm births were similarly distributed. The implication is that a factor common to women with depression who elect continuous treatment or remain unmedicated throughout pregnancy is related to preterm birth. The underlying depressive disorder and its sequelae may constitute a long-term disease risk factor independent of current symptom level. An alternative explanation is that factors independently related to active depression and SSRI treatment during pregnancy are associated with preterm birth and that the effects are of the same order of magnitude. The observation that partial exposure to SSRIs or depression was not associated with a higher rate of preterm births suggests that disease management choices related to the chronicity or severity of depression affect reproductive outcome. These data do not support a recommendation for partial SSRI treatment during pregnancy to reduce the risk of preterm birth.
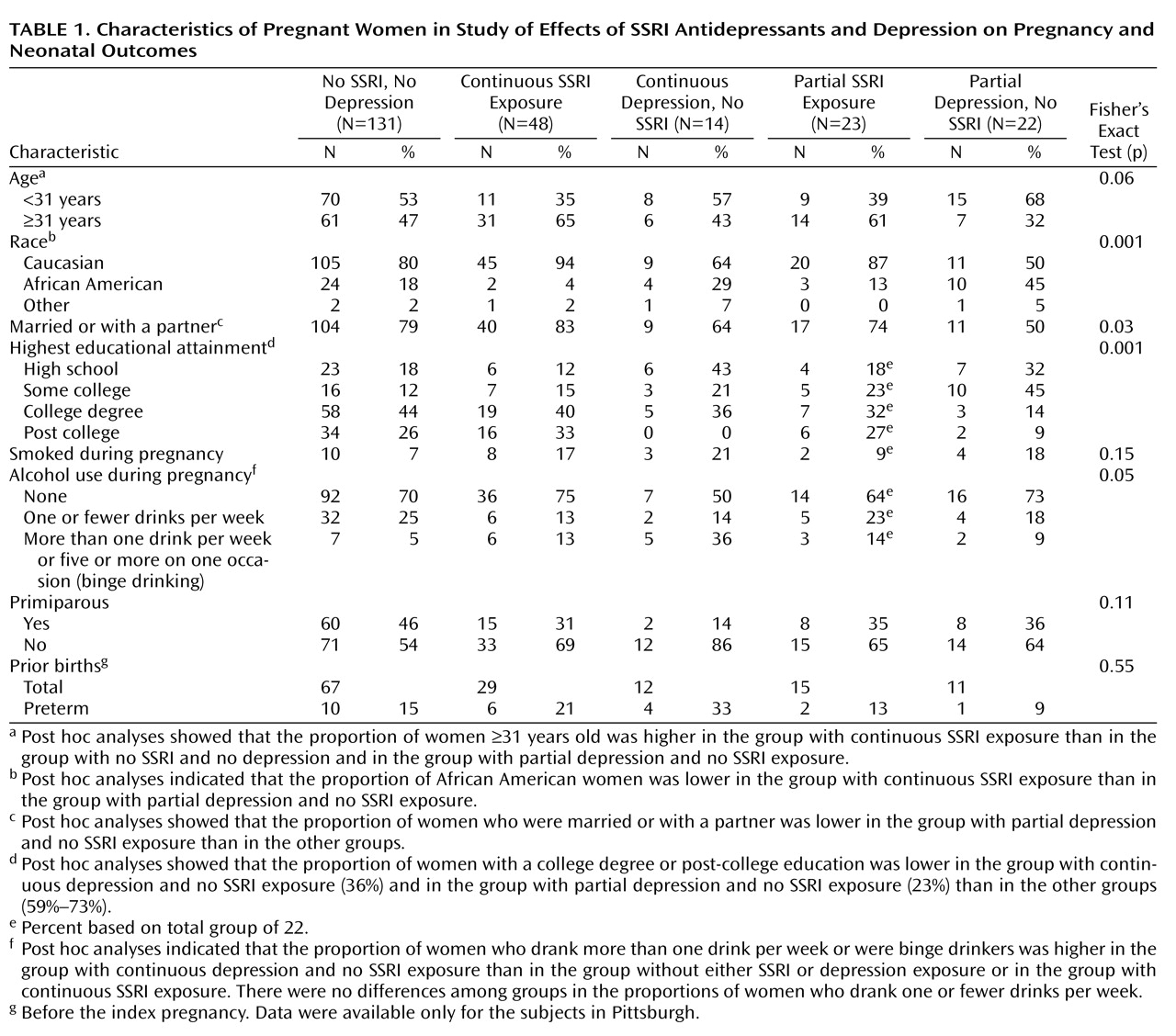
Demographic characteristics also differed across the five exposure groups. African American women were significantly more likely to have continuous depression and to be unmedicated. Racial disparities in reproductive outcomes, including preterm birth, are well described. Women with continuous SSRI exposure tended to be older, Caucasian, married, and more educated. Caucasian race, maternal age greater than 25 years, and education beyond high school are predictors for antidepressant use during pregnancy
(38) .
The majority of the recent increase in preterm births in the United States is attributable to late-preterm neonates, with the mortality rate similar to that for early-preterm infants. The relationship of preterm birth to depression and SSRI exposures must be clarified. Categorization of preterm births as due to obstetrical complications versus spontaneous preterm labor or membrane rupture would be an informative next step.
Contrary to our expectations, neonatal signs other than 5-minute Apgar scores did not differ between infants with continuous SSRI exposure and unexposed infants. A potential explanation for disparate findings across studies is that the study groups were treated with varying proportions of individual SSRI agents. The pharmacological characteristics of individual SSRIs result in different effects on neonatal outcomes
(22) . The substantial majority of reports of SSRI-related neonatal syndrome involve paroxetine
(22,
39), which has a short half-life, strong affinity for muscarinic receptors, and potent inhibition of serotonin reuptake. Costei et al.
(26) reported that 22% of neonates exposed to paroxetine had complications that required intensive treatment. Both serotonin withdrawal and cholinergic overdrive may occur in paroxetine-exposed newborns
(22) .
Fluoxetine, with a long half-life and active metabolite, is the second most frequent agent associated with neonatal syndrome
(22) . The highest reported rates for neonatal syndrome after in utero exposure were 31%
(25) in a group exposed only to fluoxetine and 30%
(40) among infants exposed to paroxetine (62%) or fluoxetine (20%). In a study by Oberlander et al.
(15) in which SSRI-exposed infants had feeding problems and respiratory distress, the majority of exposures were to paroxetine (44.7%) and fluoxetine (27.2%). Our group of women exposed to SSRIs continuously included only two (4%) treated with paroxetine and 10 (21%) treated with fluoxetine.
This investigation has several strengths. First, it is a prospective observational study of women with major depressive disorder who either had treatment with SSRIs or were untreated and experienced depression (both groups were divided into women with continuous and partial exposures), who were compared to a unexposed group. Second, unlike the majority of investigations, this study confirmed SSRI exposure by maternal serum assay. Third, assessments of maternal and infant outcomes were performed by blind raters. Finally, smoking rates did not differ across groups, and women with diagnosed alcohol abuse or dependence, a positive urine drug screen, or any exposure to FDA class D or X agents were excluded.
The weaknesses of this study include the difference in demographic characteristics (age, race, education, and marital status) among the exposure groups. Although adjustments for age and race were made, ideally these characteristics are equally distributed among groups through randomization, which was not ethical with this pregnant population. Second, a larger study group would have allowed us to be more accurate in dividing the SSRI group into categories based on response of the depression to treatment, although the mean scores for depressive symptoms and functioning for the group with continuous SSRI exposure supported benefit from treatment. Also, larger prospective studies would provide enough subjects to evaluate outcomes related to individual SSRIs.