Deficits in certain cognitive functions, such as working memory, are core features of schizophrenia
(1) . Alterations in the inhibitory circuitry of the dorsolateral prefrontal cortex may contribute to the impairments in working memory since optimal levels of γ-aminobutyric acid (GABA) neurotransmission in this cortical region are essential for normal working memory performance
(2,
3) . Consistent with this idea, postmortem studies have consistently shown lower expression of the mRNA for the 67-kDa isoform of glutamate decarboxylase (GAD
67 ), the principal enzyme responsible for the synthesis of GABA, and for the GABA membrane transporter 1 (GAT-1), in the dorsolateral prefrontal cortex of subjects with schizophrenia (see reference
4 for a review).
Understanding the functional significance of these presynaptic alterations requires knowledge of the expression levels of postsynaptic GABA
A receptors. GABA
A receptors are ligand-gated chloride ion channels assembled from different subunit classes that most commonly include 2α, 2β, and 1γ or 1δ subunits
(5) . Different combinations of subunits form GABA
A receptors with unique properties. For instance, GABA
A receptors containing a γ2 subunit predominantly mediate phasic inhibition, defined as the rapid and synchronous opening of synaptic receptors that result in an inhibitory postsynaptic potential
(6) . In contrast, δ-containing GABA
A receptors mediate tonic inhibition, defined as the constant activation of extrasynaptic receptors that, by increasing input conductance, reduces the probability of generating an action potential
(6) . Tonic inhibition mediated by δ-containing receptors has been described in many cell types, including neocortical pyramidal cells
(7,
8) .
Expression of GABA
A δ subunit mRNA was reported to be significantly lower in the dorsolateral prefrontal cortex of subjects with schizophrenia in two microarray studies
(9,
10) . Because δ subunits are thought to preferentially coassemble with α4 subunits in forebrain GABA
A receptors
(11,
12), lower δ mRNA levels in subjects with schizophrenia could represent a reduced complement of α
4 β
x δ GABA
A receptors in the illness. However, although α4 subunit mRNA levels were also lower by microarray in the dorsolateral prefrontal cortex of the same subjects with schizophrenia, the deficit was not correlated with that of the δ subunit
(10) . Furthermore, it is unclear what pathogenetic mechanisms give rise to lower levels of these subunits in schizophrenia.
In this study, in order to understand the nature of the apparent dissociation between lower levels of α4 and δ subunits in schizophrenia, we 1) evaluated the expression patterns of α4 and δ mRNAs in the dorsolateral prefrontal cortex of a larger cohort of subjects with schizophrenia and 2) explored the relationship between, and the determinants of, α4 and δ subunit mRNA expression in multiple animal models.
Results
Specificity of Human Riboprobes
The specificity of the riboprobes for the α4 and δ subunits was confirmed by several findings. First, clusters of silver grains were present over neurons, but not over glia, which are known not to express these transcripts (see Figure S1A, C in the online data supplement). Second, signal above background was not detected in tissue processed with sense riboprobes for α4 or δ subunits (see Figure S2B, D in the online data supplement). Third, consistent with previous reports in primates and humans
(17,
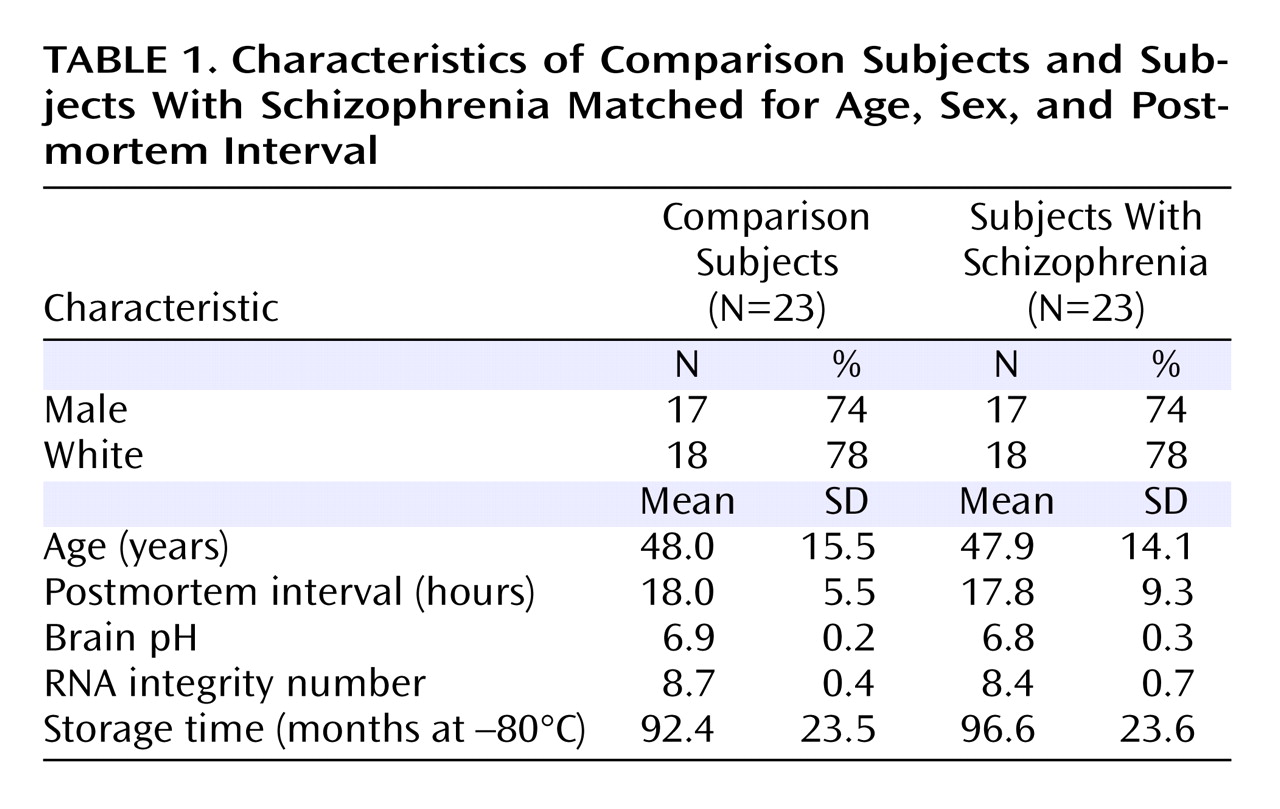
18), the expression of α4 mRNA was uniform across layers 2–5, lower in layer 6, and absent in layer 1 (
Figure 1 A). Similarly, the laminar distribution of δ mRNA was consistent with that previously described in human cortex
(18) : high and uniform across layer 2 to layer 4, low in layer 5, moderate in layer 6, and absent in layer 1 (
Figure 1 C).
Levels of a4 and d mRNAs in Schizophrenia
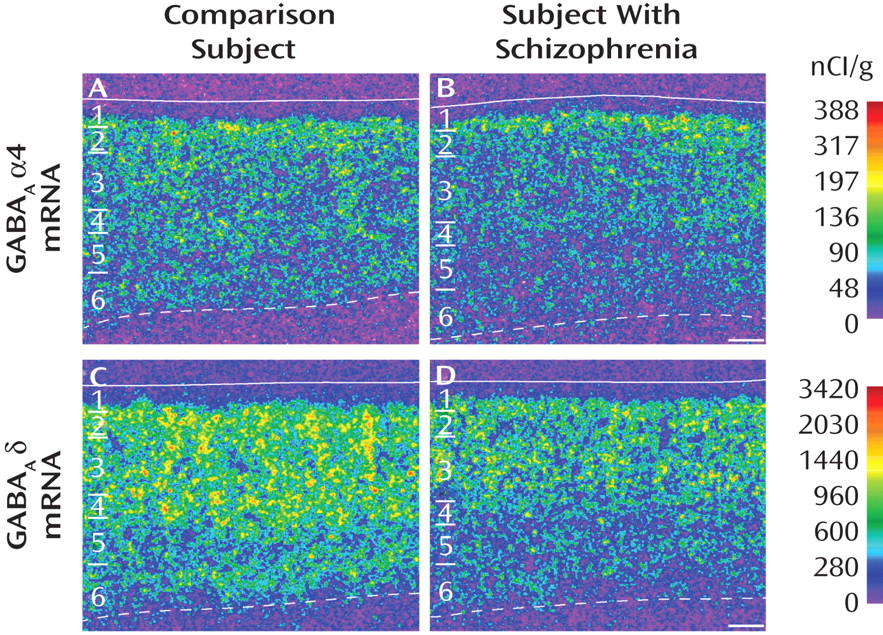
The mean α4 mRNA expression level in area 9 was only 9% lower in subjects with schizophrenia (31.2 nCi/g [SD=7.0]) than in matched comparison subjects (34.6 nCi/g [SD=8.5]), and this difference did not reach statistical significance (F=3.42, df=1, 38, p=0.072) (
Figure 2 A). In contrast, the mean expression level of δ mRNA (
Figure 2 B) was significantly (F=15.95, df=1, 38, p<0.001) lower by 19% in the schizophrenia subjects (238.15 nCi/g [SD=63.29]) than in comparison subjects (295.13 nCi/g [SD=52.96]) (
Figure 2 B).
The ANCOVAs also revealed a significant effect of age (F=19.56, df=1, 38, p<0.0001) on δ mRNA expression levels. Interestingly, levels of δ mRNA were significantly negatively correlated with age in both comparison subjects (r=–0.48, p<0.001) and schizophrenia subjects (r=–0.30, p=0.007), with the regression line in the schizophrenia subjects parallel to and shifted downward from that for comparison subjects (
Figure 2 C). This finding suggests that the disease-related reduction in δ mRNA levels is similar in magnitude across adult life.
Influence of Confounding Factors on α4 and δ mRNA Expression
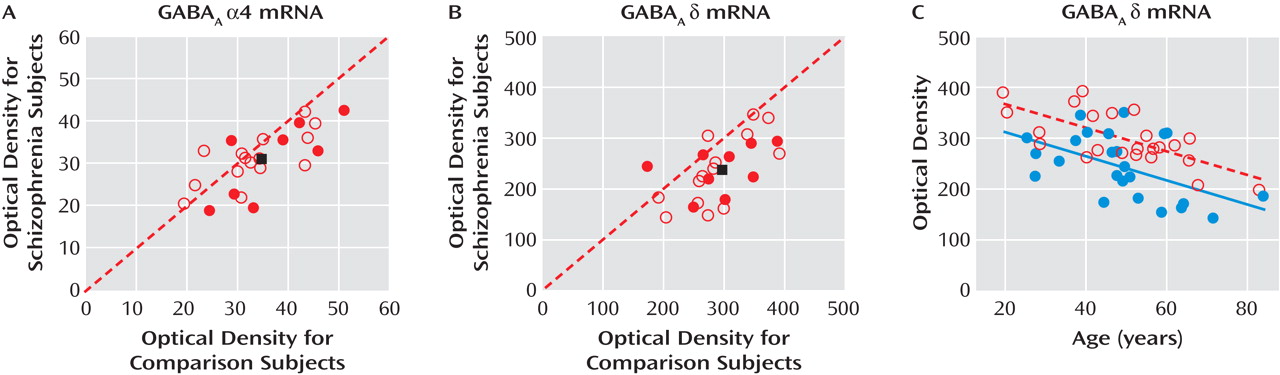
The within-subject pair differences in α4 mRNA expression were not influenced by sex, diagnosis of schizoaffective disorder, cause of death, or history of alcohol abuse or dependency (
Figure 3 A). However, the levels of α4 mRNA were decreased only in subjects with schizophrenia receiving benzodiazepines, mood stabilizers, or antidepressants at the time of death. In these subjects, the levels of α4 mRNA subunit were 16% lower relative to their matched comparison subjects. This within-subject pair difference was significantly different (t=–2.93, df=21, p=0.008) from the 4% increase in α4 mRNA levels in the subjects with schizophrenia not receiving these medications at time of death (
Figure 3 A). These findings suggest that the lower α4 mRNA levels in some subjects with schizophrenia represent a medication effect and not the disease process.
In contrast, the mean within-subject pair difference in δ mRNA expression was not influenced by sex, diagnosis of schizoaffective disorder, cause of death, alcohol abuse or dependency, or use of benzodiazepines, mood stabilizers, or antidepressants (
Figure 3 B). In addition, levels of δ mRNA in the dorsolateral prefrontal cortex did not differ among monkeys chronically exposed to haloperidol, olanzapine, or placebo (see Figure S2 in the online data supplement). Together, these findings suggest that the reduction in δ mRNA expression in subjects with schizophrenia reflects the underlying disease process.
Laminar Analysis of δ mRNA Expression in Schizophrenia
Because the lower levels of δ mRNA in subjects with schizophrenia did not seem to be a consequence of confounding factors, we assessed the expression of this transcript across cortical layers. Levels of δ mRNA were significantly lower in layer 3 (F=9.35, df=1, 38, p=0.004), layer 4 (F=10.84, df=1, 38, p=0.002), layer 5 (F=23.52, df=1, 38, p<0.0001), and layer 6 (F=10.56, df=1, 38, p=0.003), but not in layer 2 (F=1.84, df=1, 38, p=0.184), in subjects with schizophrenia (see Figure S3 in the online data supplement).
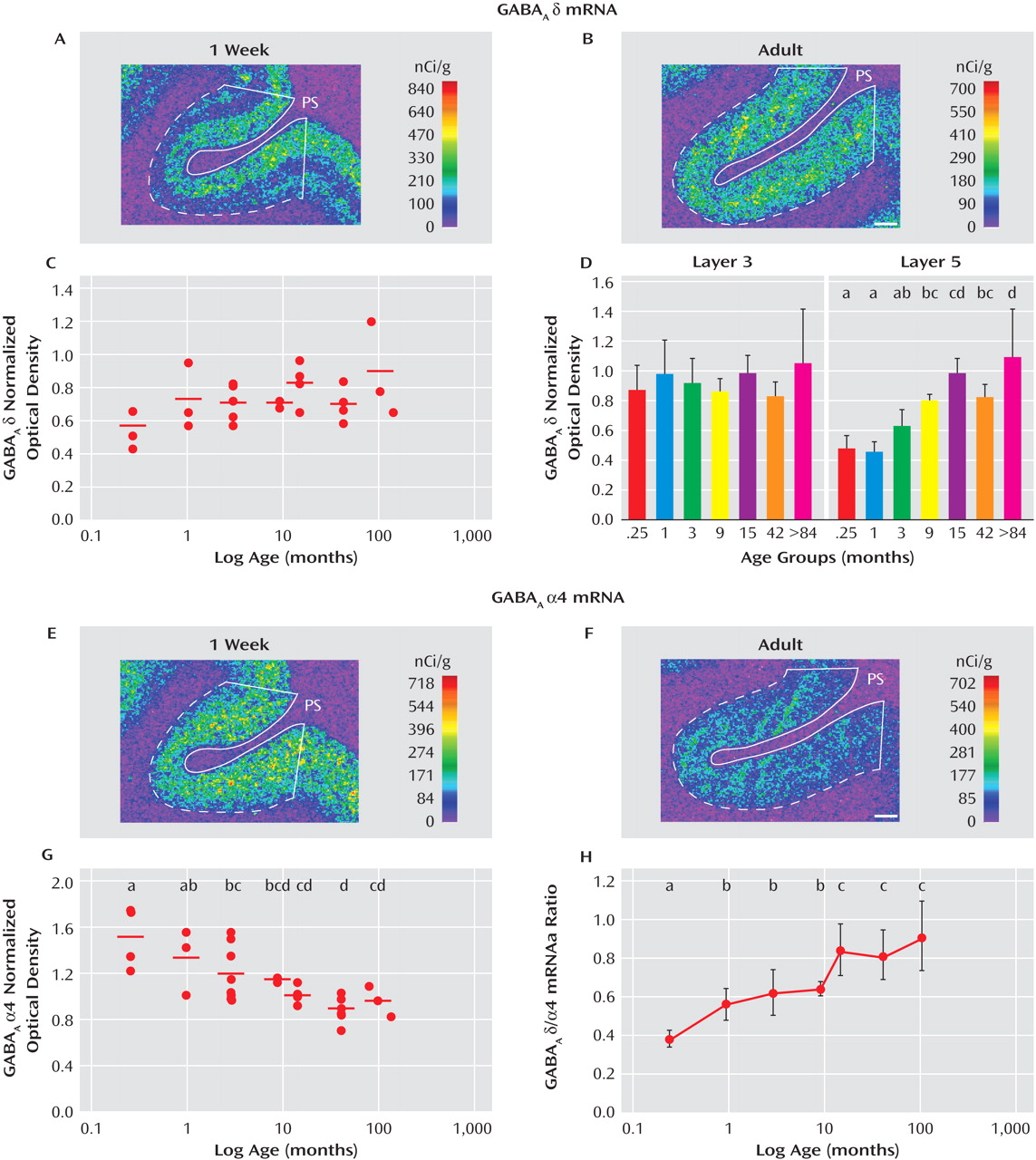
Postnatal Development of α4 and δ mRNAs in Monkey Dorsolateral Prefrontal Cortex
In order to understand the dissociation in expression of α4 and δ mRNAs in subjects with schizophrenia, we compared the postnatal developmental trajectories of these two subunits in monkey dorsolateral prefrontal cortex. The expression of δ mRNA increased during postnatal development (
Figure 4 A, B), with the mean overall cortical levels of δ mRNA 56% greater in the adult animals than those 1 week of age. However, the effect of age did not quite achieve statistical significance (F=2.45, df=6, 21, p=0.059,
Figure 4 C), perhaps because δ mRNA levels appeared to increase only in the deep layers (
Figure 4 A, B). Consistent with this interpretation, δ mRNA levels did not change with age in layer 3 but significantly (F=7.32, df=6, 20, p<0.001) increased by 116% between 1 week of age and adulthood in layer 5 (
Figure 4 D).
In contrast, the mRNA expression levels of α4 decreased during postnatal development across all cortical layers (
Figure 4 E, F). Between 1 week of age and adulthood, the mRNA levels of α4 significantly (F=5.64, df=6, 21, p=0.0012) decreased by 36% (
Figure 4 G). The opposing trajectories of α4 and δ mRNAs resulted in a marked change in the ratio of δ to α4 mRNA levels across development. Between 1 week of age and adulthood, the ratio of δ to α4 mRNA levels significantly (F=4.80, df=6, 21, p=0.003) increased by 199% (
Figure 4 H).
The mRNA expression levels of δ and α4 subunits in the sexually mature monkeys did not seem to be affected by levels of gonadal steroids or stage of menstrual cycle since among the 42-month-old monkeys, the two animals in luteal phase at the time of euthanasia had mean optical density measures for δ (0.68) and α4 (0.83) that were very similar to those of the four animals in the follicular phase (d, 0.69; α4, 0.89).
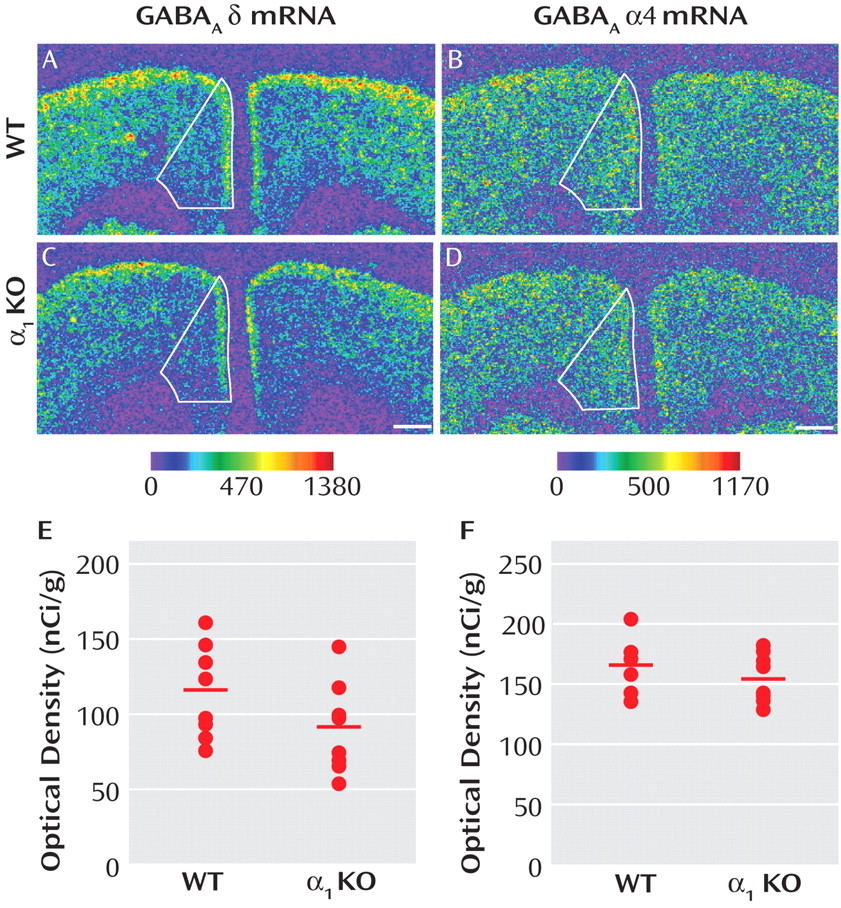
Expression of δ mRNA in Medial Frontal Cortex of GABA A α1 Subunit Knockout Mice
Reports that GABA
A receptor α1 subunit mRNA levels are significantly lower in the dorsolateral prefrontal cortex of subjects with schizophrenia
(10) and that variants in the α1 subunit gene are associated with both schizophrenia and altered expression levels of GABA
A receptor subunits
(19) suggest that lower expression of the δ mRNA in schizophrenia could be a consequence of lower expression of the α1 subunit. As a proof-of-concept test of this hypothesis, we asked whether δ subunit mRNA levels were significantly reduced in the medial frontal cortex of GABA
A α1 knockout mice. Mean δ mRNA levels in this region (
Figure 5 E) were significantly (F=10.50, df=1, 13, p=0.006) decreased by 21% in α1 knockout (104.79 nCi/g [SD=19.85]) compared with wild-type mice (131.92 nCi/g [SD=16.75]). In contrast, the mean level of α4 mRNA expression (
Figure 5 F) was not significantly different in knockout mice (155.50 nCi/g [SD=21.10]) compared with wild-type mice (166.45 nCi/g [SD=21.46]).
These findings suggest that lower δ mRNA expression in schizophrenia might be due to lower levels of α1 subunits. Consistent with this idea, the mean within-pair percent change of δ mRNA in subjects with schizophrenia as measured in our study was significantly correlated with that of α1 mRNA recently measured in the same subjects (r=0.74, p<0.0001)
(20) .
Expression of δ mRNA in Animal Models of Reduced Excitatory Neurotransmission
The expression of δ subunit also appears to be modulated by levels of excitatory neurotransmission, at least in some brain regions
(21,
22) . These findings are of interest given the evidence suggesting that schizophrenia is associated with a reduction in excitatory neurotransmission through NMDA receptors
(23,
24) . To test the hypothesis that chronically decreased excitatory signaling through NMDA receptors leads to lower δ mRNA levels, we measured the expression of δ mRNA in the medial frontal cortex of mice with reduced expression of the obligatory Nr1 subunit of the NMDA receptor (Nr1 hypomorphic mice). Genotype had a significant (F=13.44, df=2, 11, p=0.002) effect on levels of NMDA Nr1 mRNA in this brain region (see Figure S4A–C, G in the online data supplement). The mean expression levels of the NMDA Nr1 subunit were significantly (p<0.05) lower by 45% in the medial frontal cortex of mice with a
Nr1 neo/neo genotype (569.4 nCi/g [SD=50.9]) compared with wild-type mice (1043.1 nCi/g [SD=197.8]) (see Figure S4G in the online data supplement). As previously reported
(25), Nr1 mRNA levels were not significantly decreased in mice with a
Nr1 neo/+ genotype (897.7 nCi/g [SD=106.0]). However, Nr1 genotype had no effect on the expression of δ mRNA (see Figure S4D–F, G in the online data supplement). Furthermore, the mRNA levels of δ and of NMDA Nr1 were not correlated across all animals.
We also assessed whether δ subunit mRNA levels were altered in the medial frontal cortex of two rat models of reduced excitatory synaptic inputs in this brain region: adult rats with peripubertal lesions of the mediodorsal thalamic nucleus (MDTNL) and adult rats with neonatal lesions of the ventral hippocampus (NVHL). The mean mRNA levels of d subunit in the medial frontal cortex of MDTNL rats (right side: 76.3 nCi/g [SD=11.9]; left side: 75.1 nCi/g [SD=12.9]) did not differ significantly from those in animals with a sham lesion (right side: 73.8 nCi/g [SD=13.1]; left side: 72.6 nCi/g [SD=13.5]) (see Figure S5A in the online data supplement). Similarly, the mean mRNA levels of the δ subunit in the medial frontal cortex of NVHL animals (right side: 38.6 nCi/g [SD=13.5]; left side: 42.3 nCi/g [SD=8.4]) did not differ significantly from those of animals with a sham lesion (right side: 40.5 nCi/g [SD=5.6]; left side: 42.3 nCi/g [SD=6.6]) (see Figure S5B in the online data supplement).
Discussion
Our findings indicate that expression levels of the mRNA for the δ, but not for the α4, subunit of the GABA
A receptor are significantly lower in the dorsolateral prefrontal cortex of subjects with schizophrenia. To the extent that α4 mRNA levels are lower in schizophrenia
(10), the reduction appears to be due to an effect of treatment with benzodiazepines, mood stabilizers, or antidepressants at the time of death; however, we cannot exclude the possibility that lower levels of α4 mRNA reflect a particular disease process in a subtype of schizophrenia with clinical features that require the prescription of these medications.
In contrast, lower δ mRNA levels in schizophrenia, which are consistent with two microarray studies in smaller subject cohorts
(9,
10), appear to reflect the disease process of schizophrenia and are not attributable to potential confounding factors such as sex, diagnosis of schizoaffective disorder, suicide, alcohol abuse or dependency, or treatment with benzodiazepines, mood stabilizers, or antidepressants at time of death. In addition, the lower level of δ mRNA does not appear to be a consequence of exposure to antipsychotic medication, since the levels of δ mRNA were unchanged in the dorsolateral prefrontal cortex of monkeys chronically exposed to typical or atypical antipsychotics. Consistent with this interpretation, the mean percentage decrease in δ mRNA levels in the four subjects with schizophrenia who were off medications at the time of death (–23%) did not differ significantly from that in subjects who were receiving antipsychotic medications (–18%).
In contrast to rodents
(26), δ subunit mRNA levels are higher than α4 levels in human neocortex
(18) . We found that, consistent with these observations, the mean levels of δ subunit mRNA measured using probes directed at different portions of the transcript by in situ hybridization or microarray
(10) were four to nine times higher than those of α4 mRNA. Although δ subunits are thought to preferentially coassemble with α4 subunits in forebrain GABA
A receptors
(8,
11), these findings, in concert with the differential disease and developmental effects on δ versus α4 subunits, converge on the idea that the lower mRNA levels of δ subunit in subjects with schizophrenia do not reflect a reduced complement of α
4 β
x δ GABA
A receptors in the illness.
The δ subunit can coassemble with α1 subunits to form functional recombinant receptors
(27,
28), and immunoprecipitation studies have shown that δ subunits are associated with α1 subunits
(29) . GABA
A α1 subunits have also been found extrasynaptically
(30,
31), consistent with the typical localization of δ-containing receptors
(6,
32), and interneurons in the dentate gyrus exhibit immunoreactivity for α
1 and δ subunits along the cell body surface and proximal dendrites
(33) . Consistent with these observations, we found that δ mRNA expression increased across postnatal development of the monkey dorsolateral prefrontal cortex, paralleling the previously reported increase in α1 mRNA in the same animals
(34), whereas levels of α4 mRNA decreased across postnatal development. These findings support the idea that δ-containing GABA
A receptors in the adult dorsolateral prefrontal cortex preferentially coassemble with α1 subunits. Furthermore, unlike in the rodent hippocampus
(33), GABA
A receptors containing both δ and α1 subunits are likely to be found principally in pyramidal cells in the primate neocortex. For example, a previous study of postmortem human motor cortex
(18) revealed that δ subunit mRNA clustered mainly over pyramidal cells. Consistent with this finding, our analysis of δ mRNA silver grains indicated that in the human dorsolateral prefrontal cortex, δ mRNA expression was also clustered primarily over putative pyramidal cells, characterized by their faint Nissl staining and large nuclei (see Figure S1 in the online data supplement). Therefore, the δ subunit mRNA appears to be predominantly expressed by cortical pyramidal cells, although we cannot exclude a lower level of expression in GABA-containing interneurons. This interpretation is further supported by the absence of δ mRNA expression in layer 1 of the dorsolateral prefrontal cortex (
Figure 1 ), which contains GABA-containing interneurons but not pyramidal cells.
Thus, lower levels of δ mRNA in schizophrenia might reflect a reduced complement of α
1 β
x δ GABA
A receptors in dorsolateral prefrontal cortex pyramidal neurons. Consistent with this interpretation, the differences in α1
(20) and δ mRNA levels were significantly correlated as measured by in situ hybridization (r=0.74, p<0.0001) in the subjects used in this study and as measured by microarray (r=0.81, p<0.001) in a previous study involving a subset of the subjects studied here
(10) . However, further studies are needed to determine whether the lower levels of α1 and δ subunit mRNAs in the dorsolateral prefrontal cortex of subjects with schizophrenia are paralleled by lower levels of their cognate proteins and assembled GABA
A receptors.
The lower mRNA levels of δ subunit in schizophrenia might be a consequence of lower levels of α1 subunit, given that δ mRNA levels are significantly reduced in the medial frontal cortex of α1 knockout mice. However, it should be noted that δ mRNA levels were unchanged in other brain regions of α1 knockout mice
(35) . Thus, it is unclear whether the potential effect of lower α1 subunits on the mRNA expression levels of δ is limited to certain cortical regions. Although it has been suggested that the disruption of excitatory signaling through NMDA receptors could produce the alterations in presynaptic markers of GABA neurotransmission seen in schizophrenia
(24), δ mRNA levels were not altered in three animal models with two forms of reduced excitatory drive in the medial frontal cortex: 1) reduced number of NMDA receptors and 2) reduced presynaptic excitatory inputs from the thalamus or hippocampus. Thus, these findings do not support the hypothesis that a chronic reduction in the activity of excitatory inputs represents a pathogenetic mechanism resulting in lower expression of δ subunit mRNA in subjects with schizophrenia.
Alternatively, lower levels of δ-containing GABA
A receptors (and potentially of tonic inhibition) in schizophrenia might represent a compensatory response to presynaptic reductions in GABA neurotransmission. Release of GABA provides inhibitory control over postsynaptic cells via both synaptic and extrasynaptic GABA
A receptors. An important functional role of synaptic receptors, which mediate phasic inhibition, is the generation of rhythmic activities in neuronal networks
(6) . The resulting synchronized activity gives rise to network oscillations that are thought to contribute to cognitive processes, such as working memory. For instance, the synchronized firing of neuronal networks at 30–80 Hz, known as gamma band oscillations, is induced and sustained in the human dorsolateral prefrontal cortex during working memory tasks
(36,
37) . Thus, lower levels of prefrontal GAD
67 mRNA, leading to a deficit in GABA synthesis and impaired phasic inhibition, have been proposed to be a substrate for reduced frontal lobe gamma band power and working memory deficits in schizophrenia
(38,
39) . Although the role of extrasynaptic receptors and tonic inhibition in the generation or maintenance of oscillations is less clear, a decrease in tonic inhibition could represent a compensatory response since mutant mice with a complete loss of tonic inhibition in the hippocampus exhibit an increase in the power of gamma band oscillations
(40) .