Patients with major depressive disorder often report self-deprecating thoughts and feelings of inadequacy and worthlessness (
1–
3). Such negative thoughts come to mind without effort or conscious desire, interfere with other cognitive processes, and are not easily changed. This distorted negative view of the self has long been considered a hallmark feature of depression, observed by clinicians and theorized to be a contributing factor in the etiology and manifestation of the disorder (
4–
9). It is hypothesized that individuals in a current major depressive episode or those at risk for the disorder have predominantly negative self-schema, which are considered to be automatic because they come to mind without conscious effort or desire and are hard to inhibit. Such self-schema may be dormant and unexpressed prior to the onset of depression or during remission but usually become active during an acute episode (
4,
5).
Although the theory emphasizing the role of automatic negative self-views in depression has gained wide acceptance among clinicians, attempts to empirically substantiate this theory have been inconclusive. Studies examining automatic emotional processes often fail to find a bias for negative information. In contrast, there is consistent evidence supporting the role of strategic (i.e., effortful and elaborative) processing of negative information related to the self (
3,
10–
14). For example, currently depressed patients exhibit preferential memory for negative words, mainly when they are asked to specifically focus on the semantic meaning of these words (i.e., strategic encoding) rather than the perceptual features (i.e., automatic encoding). These findings influenced an alternative conceptualization of the nature of cognitive deficits in patients with major depression in a study conducted by Williams and colleagues (
3), who posit that greater depth of processing may account for preferential memory for negative self-referent information (
15).
Moreover, it remains unclear whether self-referent automatic negative thoughts represent cognitive vulnerability for depression. Behaviorally, negative biases are most often found during a major depressive episode rather than prior to it or following recovery (
16–
19). However, negative self-schema in individuals in remission or at risk emerge when the eliciting tasks capitalize on automatic rather than effortful processes (e.g., indirect tests of memory, such as priming [
20,
21]).
Given this mixed pattern of results, we propose an integrative model. Specifically, we believe that the automaticity of access to and activation of negative self-schema in depression may be established and reinforced through effortful elaboration and rumination. In turn, automatic activation of negative self-schema may trigger further elaboration and rumination. Automatic reactivation of previously established self-concepts may be augmented or superseded by state-dependent effortful elaboration and rumination. Thus, mood-congruent changes in effortful processes (e.g., shifts in focus from positive to negative information) are likely to contribute to the manifestation of negative self-referential biases in depression. In particular, cognitive vulnerability reflected in abnormal automatic processes may be unexpressed in behavioral tasks during remission, compensated for by an active suppression of negative information or healthy strategic elaboration of positive information (
22). During an episode of depression, the effectiveness of compensatory effortful processes may diminish and be replaced by increased self-focused rumination and elaboration of negative information, resulting in the emergence of negative biases (
22,
23).
Although existing studies provide partial support for this theory, their results are not conclusive given the limited ability of behavioral tasks to isolate automatic and effortful processes (
24–
26). In the present study, we used event-related brain potentials to examine the hypothesized contributions of trait-specific abnormalities in automatic processes and state-dependent abnormalities in effortful processes to self-referential biases in current and remitted major depression.
Event-related brain potentials are well-established indices of cognitive and neural resource allocation during specific cognitive stages (
27,
28). Automatic processes were indexed by the P2 component, which is thought to reflect the ongoing automatic monitoring of semantic meaning and significance of incoming information (
29). Linked to attentional capture, the P2 component is one of the earliest components known to be modulated by valence (
30). Effortful processes (e.g., elaboration on the semantic meaning of stimuli) were indexed by the late positive component, which reflects greater effort and resource allocation during strategic cognitive processes, such as categorization, elaboration, and semantic encoding (
31,
32).
We examined P2 and late positive component amplitudes during memorization/encoding stages of an intentional recall task, during which participants indicated whether a word described them (i.e., self-referential condition) or, in a different set, whether a word described former President Bill Clinton (i.e., other-referential control condition), who was chosen because of the wide familiarity and strong emotional responses elicited by his persona. We decided against using a "close other" condition (e.g., mother), since such categorization appears to rely on a knowledge base (i.e., schema) similar to that of self-referential processing (
33).
Method
Participants
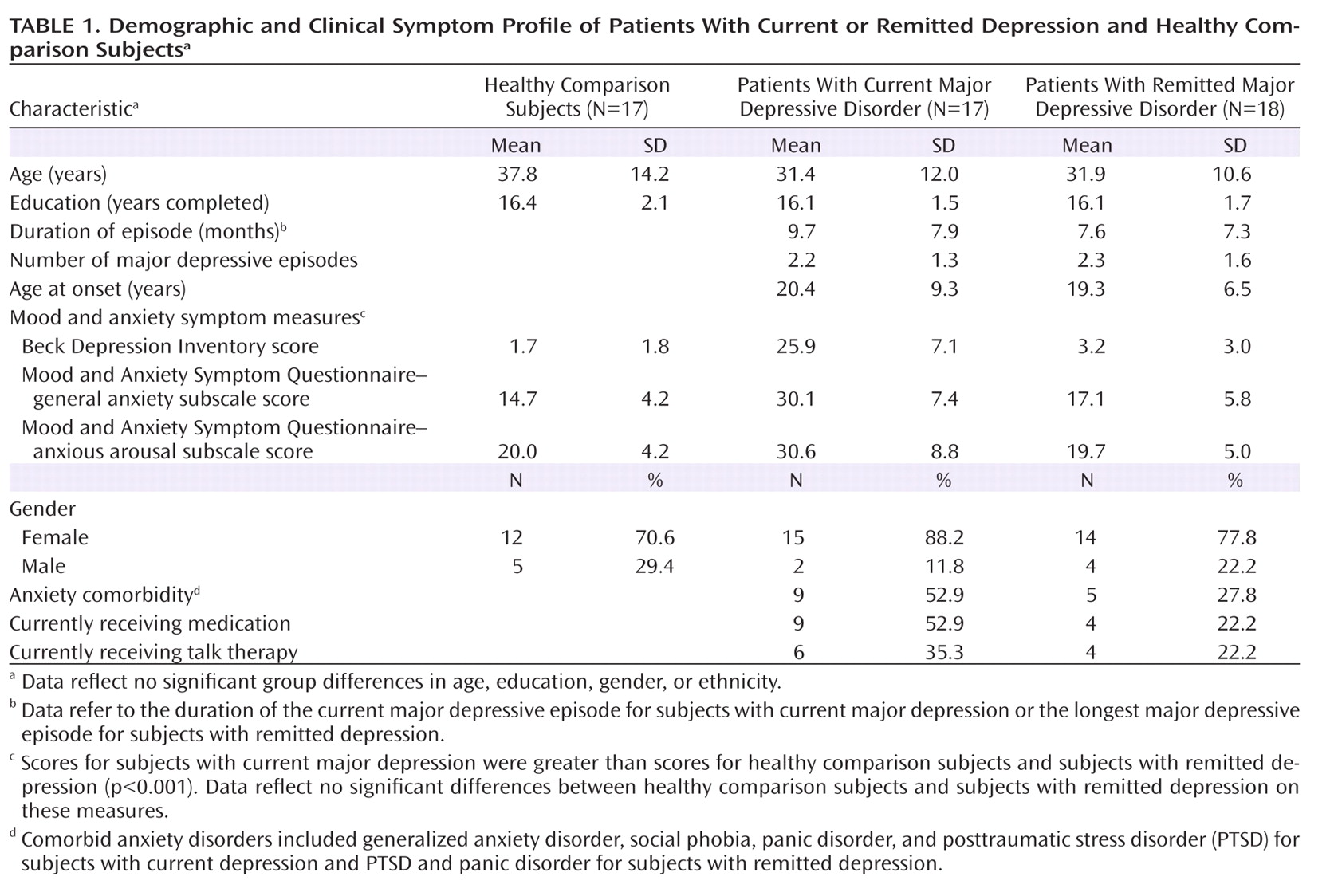
Participants were recruited from the general population of the Greater Boston area. Individuals in the current depression group met DSM-IV criteria for a current major depressive episode per the Structured Clinical Interview for DSM-IV. Individuals in the remitted depression group met diagnostic criteria for a past but not current major depressive episode, had no residual symptoms, and had a Beck Depression Inventory (
34) score <7 for at least 1 month prior to testing. Participants who did not meet criteria for any current or past axis I disorder were included in the comparison group (
Table 1).
Exclusion criteria were as follows: current or past psychotic or manic symptoms, substance abuse/dependence, attention deficit hyperactivity disorder, current pregnancy or menopause, history of ECT, brain injury, and any medical illnesses that may cause neurological or hormonal abnormalities. Individuals with past major depression were excluded if they met diagnostic criteria for any current anxiety disorder within the past 2 months. Potential effects of anxiety symptoms on behavioral and physiological data were examined in correlation analyses using general anxiety and anxious arousal subscales of the Mood and Anxiety Symptom Questionnaire (
35).
Participants were also excluded if they modified their medication regimen in the past 3 weeks, and only those who still met diagnostic criteria for a major depressive episode despite taking medications (selective serotonin reuptake inhibitors) were included in the current depression group. Medication status (i.e., medicated or unmedicated) was entered as a factor into analyses, and, given no significant effects, data for medicated and unmedicated participants were pooled together.
Procedure
During the diagnostic interview, participants received detailed study information, provided informed consent, and were scheduled for the physiology session, which took place 2–5 days later. During the physiology session, EEG activity was recorded while participants performed an identification task under the following two encoding conditions: 1) self-referential, in which they indicated whether each adjective word could be used to describe them, and 2) other-referential, in which they indicated whether each word could be used to describe former President Bill Clinton. Subjects responded by pressing a "yes" or "no" button, with the response hand (left, right) counterbalanced within and between groups.
Words were presented in five blocks of 18. Each word was displayed on the screen for 200 msec, followed by a crosshair displayed for 10 seconds, during which time participants made their responses. At the end of each block, subjects were asked to count backward from 50 to one out loud for 1 minute to prevent maintenance rehearsal. After this interference period, subjects were given a sheet of paper and asked to write down words they remembered from the previous block. Between the self-referential and other-referential encoding conditions, participants were engaged in one of several different randomly chosen 20-minute filler tasks (e.g., rating pictures or sounds).
Stimuli
Forty-four positive and negative adjectives were selected from the Affective Norms for English Words based on provided normative ratings of valence and arousal (
36) and word frequency (
37). Positive and negative stimuli were significantly different for valence (p<0.01) but not arousal, word frequency, or length. Stimuli were equally distributed and pseudorandomly presented within each block, with no more than two words of the same valence or arousal presented in a row. The same stimuli sets were presented in both encoding conditions, with the order of words in each condition and the presentation order of conditions counterbalanced within and between the groups to account for practice effects.
Apparatus and Physiological Recording
EEG signals were recorded using a cap with Ag/AgCl sintered electrodes (Electro-Cap International, Inc., Eaton, Ohio) from the left, midline and right frontal, fronto-central, central, centro-parietal, and parietal sites. Electro-oculography was used to record horizontal and vertical eye movements using electrodes placed on the outer canthi and right sub- and supraorbital positions. Electrogel (Quik-Gel, Neuromedical Supplies, Inc., Herndon, Va.) was used to ensure proper conductivity. Electrode impedances were kept under 5 KΩ.
Signals were measured with reference to the left mastoid (M1) and re-referenced offline to the mastoid average. A 37/64-channel custom bioamplifier (S.A. Instruments, San Diego) was used to amplify, sample (3 kHz), and filter (high-pass: 0.01 Hz; low-pass: 100 Hz) physiological data. Signals were digitally filtered offline with a low-pass 30-Hz filter and resampled at 128 Hz. Eye-movement artifacts were corrected using James Long Company software (Caroga Lake, N.Y.), with visual confirmation of the success of artifact correction. Trials involving residual eye-blink and muscle-movement artifacts were excluded. EEG data for valid trials were averaged by electrode site for each participant within each encoding and valence condition within the 3-second poststimulus time window relative to a 250-msec prestimulus baseline. The P2 component was quantified as a positive peak in the 200- to 300-msec time window poststimulus, and the late positive component was quantified as the average voltage in the 600- to 800-msec time window poststimulus.
Data Reduction and Statistical Analyses
Behavioral data
The number of recalled words, reaction times, and endorsement data were examined using 3×2×2 repeated-measures analyses of variance, with group (current depression, remitted depression, or comparison) as the between-group factor and valence (negative versus positive) and encoding condition (self- versus other-referential) as the within-group factors. To examine whether memory biases for emotional information may be accounted for by the corresponding bias in the endorsement of emotional words as descriptive of the self or others, the number of "yes" responses in each valence and encoding category was correlated with the number of remembered words in the corresponding valence and encoding category in each group.
Physiological data
Amplitudes for the P2 and late positive component were subjected to 3×2×2×5×3 repeated-measures multivariate analyses of variance, with group (current depression, remitted depression, or comparison) as the between-group factor and encoding condition (self- versus other-referential), valence (negative versus positive), caudality (frontal, fronto-central, central, centro-parietal, or parietal), and laterality (left, midline, or right) as the within-group factors. Significance values were calculated using Greenhouse-Geisser correction.
Results
Behavioral Data
Free recall
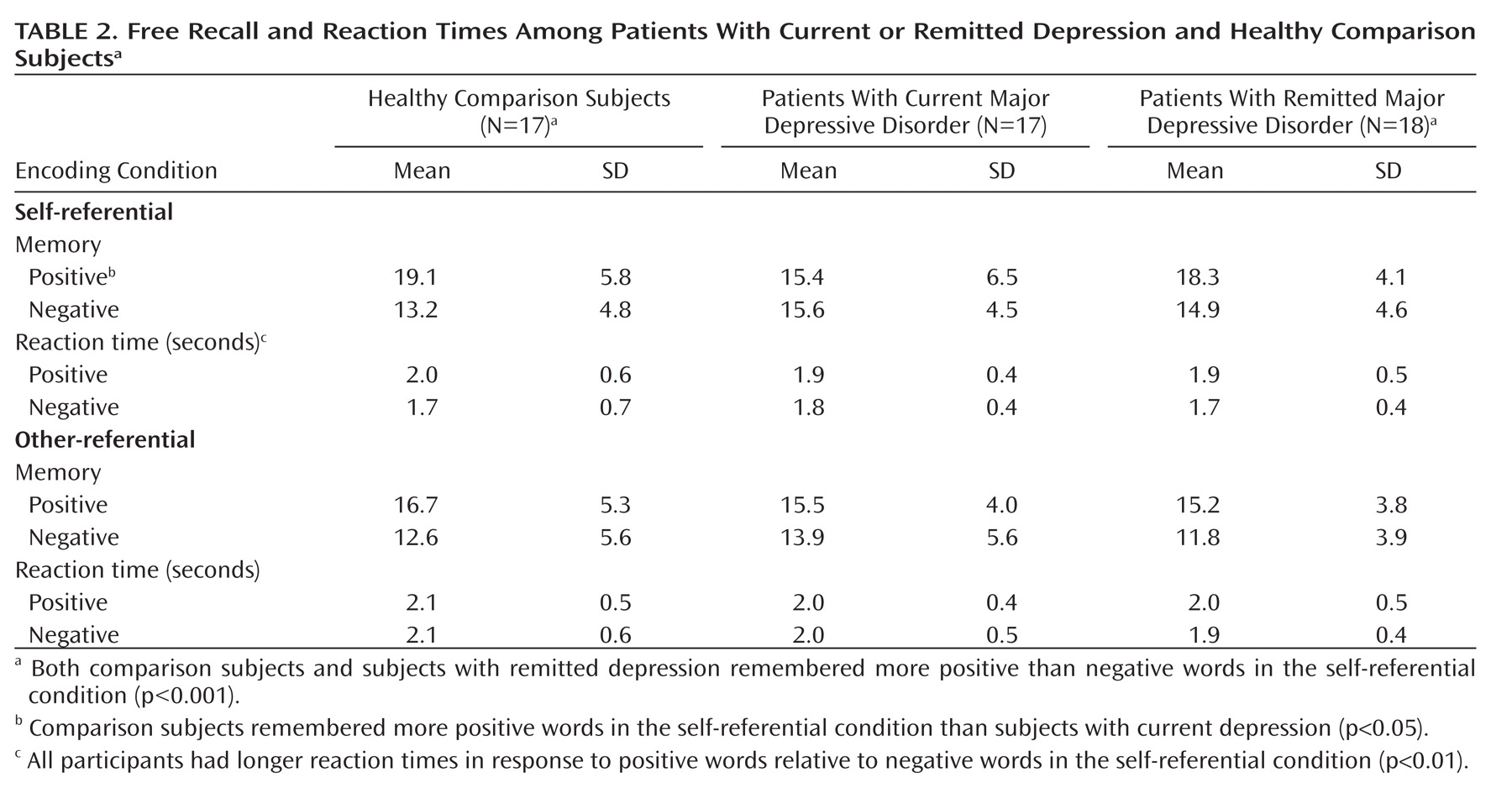
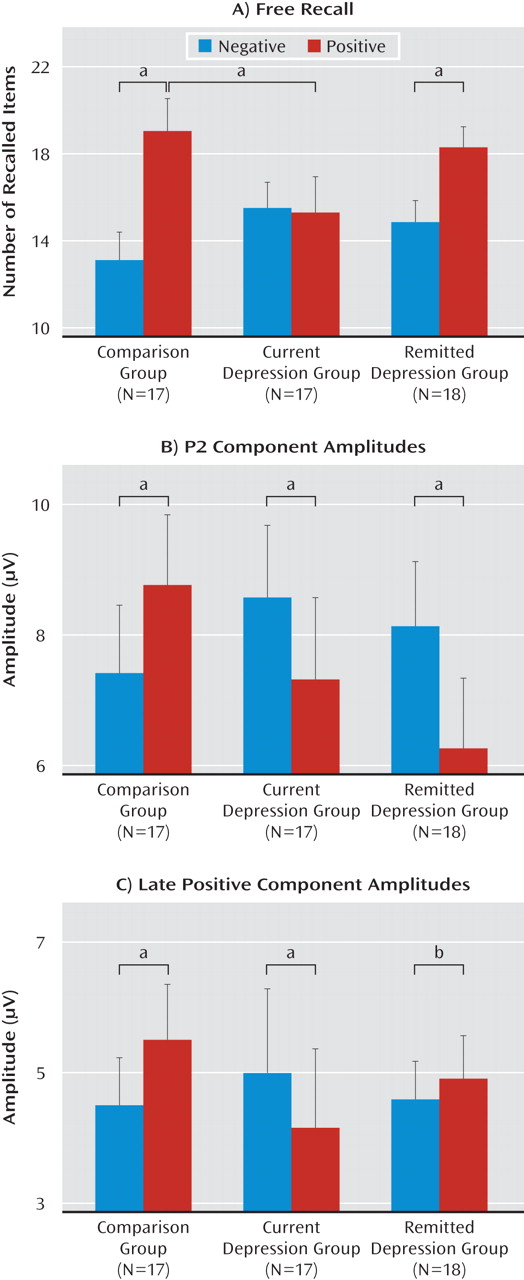
Participants remembered more words in the self-referential encoding condition than in the other-referential encoding condition (F=14.6, df=1, 49, p<0.001). The three groups exhibited significant differential memory for emotional stimuli in the self-referential condition only (valence-by-group: F=10.9, df=2, 49, p<0.001) (
Table 2,
Figure 1). Specifically, comparison subjects remembered more positive words than subjects in the current depression group (p<0.05). Comparison subjects (p<0.001) and subjects with remitted depression (p<0.001), but not current depression, exhibited greater memory for positive stimuli relative to negative stimuli. Individuals with current depression remembered as many positive as negative words in the self-referential encoding condition. Among these individuals, the number of remembered positive stimuli correlated with the general anxiety (r=0.55, p<0.05) and anxious arousal (r=0.49, p<0.05) subscales of the Mood and Anxiety Symptom Questionnaire.
Endorsement of stimuli as self- or other-referential
Within the self-referential encoding condition, the three groups exhibited significant differences in the endorsement of positive and negative stimuli (valence-by-group: F=84.1, df=2, 49, p<0.001). Specifically, both comparison subjects (p<0.01) and subjects with remitted depression (p<0.01) endorsed more positive than negative words, whereas participants with current depression endorsed more negative than positive words (p<0.01). Comparison subjects and subjects with remitted depression endorsed more positive words (p<0.001) and fewer negative words (p<0.001) than subjects with current depression. There were no significant differences in endorsement between subjects in the comparison and remitted depression groups.
Participants also demonstrated significant group differences in the endorsement of emotional stimuli as other-referential (valence-by-group: F=3.4, df=2, 49, p<0.05). Specifically, subjects in the comparison (p<0.001) and remitted depression (p<0.01) groups demonstrated greater endorsement of positive words relative to negative words, and there was a nearly significant difference for subjects with current depression in greater endorsement of positive words relative to negative words (p<0.07). Further, comparison participants endorsed more positive words than subjects with current depression (p<0.01).
Correlations between self-referential endorsement and recall rates revealed a nearly significant relationship between the endorsement and recall of negative words among individuals in the comparison group (r=0.49, p<0.06) and individuals with current depression (r=0.45, p<0.08).
Physiological Data
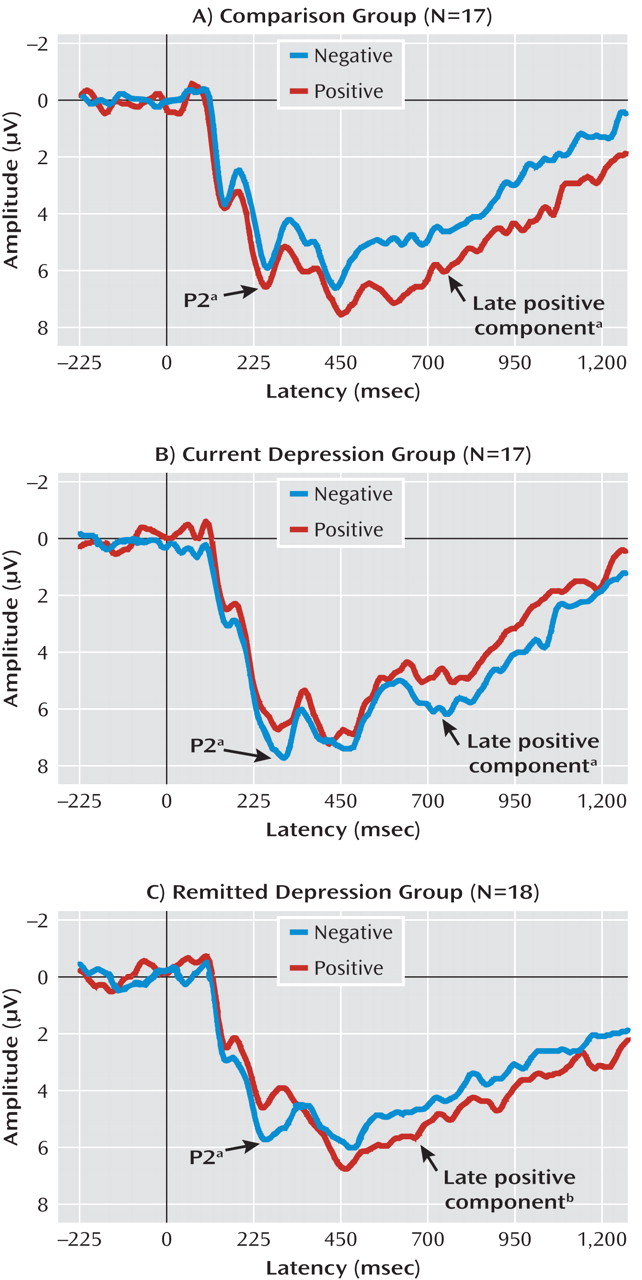
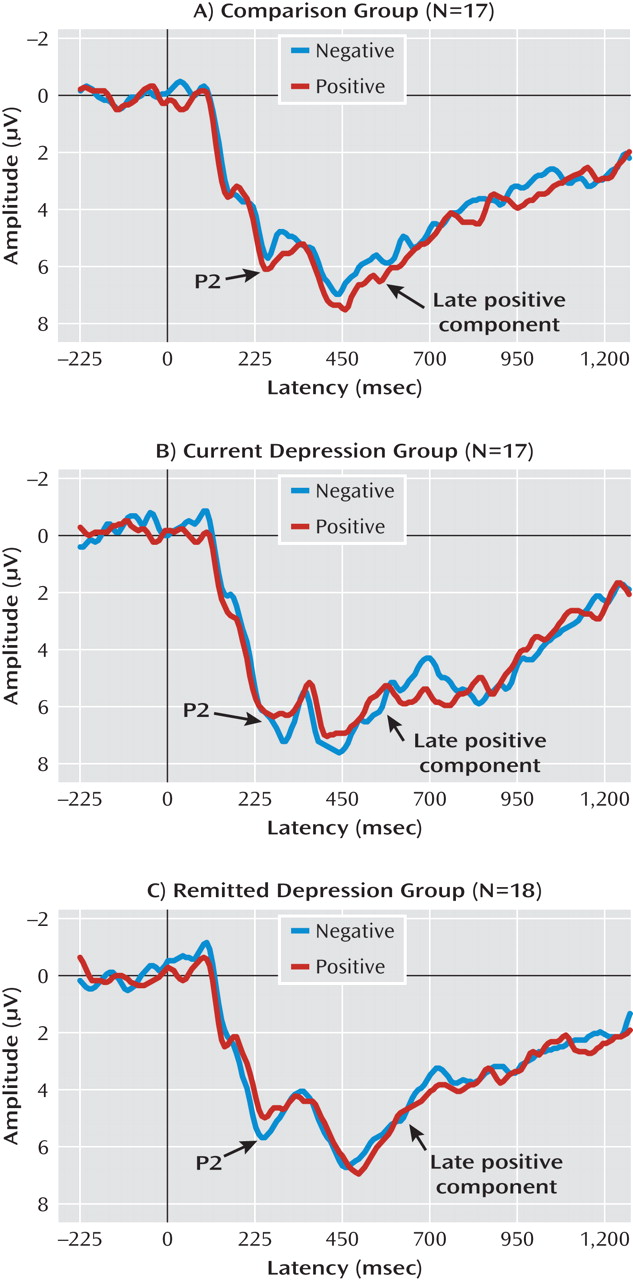
Representative average waveforms for each group in the self-referential and other-referential encoding conditions are presented in
Figure 2 and
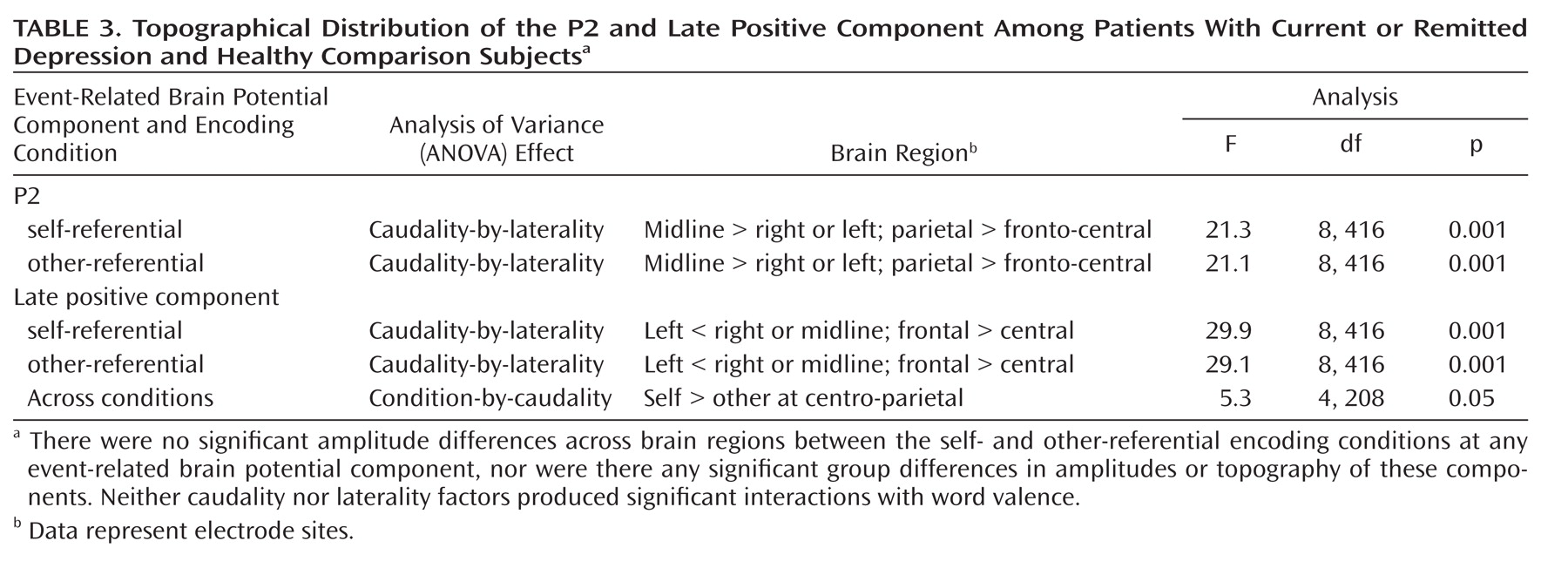
Figure 3. Topographical distribution of the event-related brain potential components is described in
Table 3.
Group differences in automatic processing of emotional stimuli
The three groups demonstrated significant differences in P2 amplitudes during early automatic self-referential, but not other-referential, encoding of emotional stimuli (task-by-valence-by-group: F=4.4, df=2, 49, p<0.05). Specifically, whereas individuals in the comparison group exhibited an increase in P2 amplitudes during encoding of positive relative to negative stimuli (p<0.05) in the self-referential condition, individuals with current (p<0.05) and remitted (p<0.01) depression exhibited greater P2 amplitudes to negative relative to positive stimuli (valence-by-group: F=9.7, df=2, 49, p<0.001).
Group differences in strategic processing of emotional stimuli
For the late positive component, the groups demonstrated significant differential amplitudes during self-referential, but not other-referential, encoding of emotional stimuli (task-by-valence-by-group: F=3.8, df=2, 49, p<0.05). Specifically, in the self-referential task, comparison subjects demonstrated an increase in amplitudes during encoding of positive relative to negative stimuli (p<0.01), subjects with current depression exhibited greater amplitudes during encoding of negative relative to positive stimuli (p<0.05), and subjects with remitted depression exhibited a nearly significant difference in greater amplitudes in response to positive relative to negative stimuli over the frontal sites (p<0.08) (valence-by-group: F=5.8, df=2, 49, p<0.01). There were no group or valence differences in late positive component amplitudes in the other-referential encoding condition.
Correlation analyses
In subjects with current depression, greater positive memory bias in the self-referential condition was associated with greater positive P2-amplitude bias at the left fronto-central site (r=0.50, p<0.05). For the late positive component, comparison subjects exhibited a direct relationship between memory performance and event-related brain potential amplitudes for positive stimuli (all r values >0.50, p<0.05). In the group with current depression, greater memory for positive and negative stimuli was associated with greater frontal late positive component amplitudes (all r values >0.50, p<0.05). There were no correlations among subjects with remitted depression, and there were no correlations between the endorsement of words as self- or other-referential and component amplitudes in either group. Finally, greater anxious arousal scores were associated with greater P2 amplitudes during processing of positive self-referent stimuli among subjects with current depression (r=0.49, p<0.05). There were no corresponding correlations in the other-referential condition.
Discussion
In the present study, we aimed to reconcile the following two largely competing theories of biased processing of self-referential information in major depression: Beck's cognitive theory (
5), which emphasizes the role of uncontrollable automatic biases toward negative information, and the empirically based model of Williams and colleagues (
3), which emphasizes the role of effortful and controlled rumination and elaboration on negative information. We proposed that abnormalities in both automatic and controlled processes may contribute to the manifestation of self-referential biases in major depression, such that automatic cognitive biases toward negative information reflect a cognitive vulnerability, whereas rumination and effortful elaboration on negative information reflect a mood-dependent feature of the disorder. Thus, preferential processing of negative information would be expected during early automatic stages of orienting and attention capture in individuals who are currently or have been previously depressed (i.e., those who possess a cognitive vulnerability for the disorder). In contrast, preferential processing of negative information during more effortful elaboration and rumination would be evident only in individuals who are currently depressed and would ameliorate or switch to a bias for positive information in those who are in remission.
Our findings mainly support the proposed hypotheses and indicate that anomalous automatic processing of emotional self-referential information represents a stable trait in individuals who have experienced at least one major depressive episode. Specifically, individuals with current or remitted depression exhibited greater P2 amplitudes, indexing automatic attentional capture and orienting, during self-attribution of negative words relative to positive words. Abnormalities in strategic elaboration of self-referential information appeared to be mood-dependent: whereas subjects with current depression exhibited greater late positive component amplitudes in response to negative relative to positive words, both comparison subjects and, to a lesser degree, subjects with remitted depression exhibited greater late positive component amplitudes in response to positive relative to negative stimuli. These results were echoed by the behavioral performance, such that comparison subjects and subjects with remitted depression remembered more positive than negative words. In turn, participants with current depression remembered as many positive as negative items. Correlations between event-related brain potentials and memory in comparison and depressed participants provide a direct connection between brain activity during automatic and strategic processing of emotional information and observed memory biases.
These findings offer compelling evidence addressing the ongoing debate regarding contributions of automatic and strategic processes and provide support for the integrative theory of the nature of dysfunctional self-oriented cognition in major depression. First, greater automatic processing of negative relative to positive self-referential words in both patient groups indicates an attentional bias toward processing negative information congruent with the self-schema and may represent a cognitive vulnerability for depression suggested by Beck (
5 [see also references
14,
21,
23]). Although it remains unclear whether the negative P2-amplitude bias was a predisposition for major depression or a result of a history of depression, it is evident that abnormal automatic processing of negative self-referential information was present, albeit latent, even in full remission.
Second, strategic elaboration of negative information also plays an important role in the manifestation of self-oriented cognitive biases and appears to be influenced by the current state. In fact, the augmented strategic processing of positive stimuli in participants in remission may have partially compensated for the initial attentional bias toward negative stimuli and may have contributed to the manifestation of positive bias at recall. These findings support cognitive vulnerability theories of depression, suggesting that automatic bias for negative information in individuals at risk may be masked by compensatory effortful elaboration on positive information when depression symptoms subside (
23). However, greater strategic processing of positive self-referential information in subjects with remitted depression was only marginally significant, indicating that the amelioration of deficits in effortful processing in remission may not be absolute, or that a small-to-moderate effect size in this group did not reach the significance threshold as a result of the limited statistical power of the study. These results suggest that specific targeted focus on positive self-referential information may be a beneficial psychotherapeutic tool in the treatment of major depression and should be emphasized in the current framework of cognitive-behavioral therapy (CBT).
The present findings are also consistent with neurobiological theories of depression suggesting that self-referential processing is implemented in the ventral and dorsal medial prefrontal cortico-limbic networks (
38). The ventral network, which also comprises the rostral anterior cingulate, insula, and amygdala, is thought to contribute to implicit appraisal of information as relevant to the self and is likely to reflect automatic self-referential processing. The dorsal network, which includes the dorso-lateral prefrontal cortex and hippocampus, is thought to implement strategic control over the initial automatic appraisals. Abnormal activation of these regions is found in individuals with major depression and appears to remediate following CBT (
39). Moreover, recalling self-relevant negative information has been previously found to elicit a decrease in ventral medial prefrontal cortex and rostral anterior cingulate activation in participants with both current and remitted depression, potentially indicating abnormal resource allocation to automatic processing of emotional information. In contrast, subjects with current but not remitted depression have been found to demonstrate greater decrease in the dorsal medial prefrontal cortex, dorso-lateral prefrontal cortex, and hippocampus, suggesting insufficient resource allocation to effortful processing of emotional self-relevant information (
40). Although contributions of these networks to amplitudes of the P2 and late positive components is speculative, it is probable that group differences in automatic and strategic processing of self-referent emotional information in the present study indicate dysfunctional activation of these networks in depression.
The observed biases in the processing of emotional words were specific to the self-referential and not other-referential conditions, even though the same words were used in both conditions. Moreover, although the observed memory biases in the self-referential state could be related to differences in endorsement of stimuli as self-referential, the group differences in event-related brain potential amplitudes could not be accounted for by endorsement differences given the lack of corresponding correlations. Finally, although some of the currently depressed individuals also met diagnostic criteria for anxiety disorders and, as a group, exhibited more anxiety, this confound does not appear to have significant interpretive implications in our study, since anxiety symptoms were related to better memory for and greater P2 amplitudes in response to positive rather than negative words. This effect is consistent with theories that emphasize differential cognitive deficits in mood and anxiety disorders and suggest that processing of positive information may be less impaired in anxiety than depression (
6,
35).
In summary, the present study provides direct evidence for an integrative theory of dysfunction in self-oriented cognition in major depression, emphasizing both mood-independent abnormalities in automatic processing and mood-dependent abnormalities in strategic processing. Although we did not address this issue directly, it is likely that biased automatic processes represent a stable marker for depression and may be compensated for by unimpaired strategic processes during normal mood. Additional investigations are required to determine whether 1) the presence of abnormalities in automatic processing of emotional stimuli is a risk factor for developing major depression, 2) these abnormalities in automatic processing can be remediated with treatment, and 3) training currently depressed individuals to use compensatory strategic elaboration of positive information reduces symptoms.
Acknowledgments
The authors thank Pearl Chiu, Christen Deveney, Anna Glezer, Brooks King-Casas, and Laura Phillips for assistance with and contributions to data collection.