Individuals with schizophrenia have pronounced episodic memory impairments
(1) that are not explained by demographic variables such as education or sex
(2) or by clinical variables, including medication, duration, or severity of illness
(3) . Deficits in unaffected first- and second-degree relatives
(4,
5) suggest that these impairments are genetically mediated. Since these deficits limit functional outcome
(6) and do not respond well to available treatments
(7), there is increased interest in identifying neural mechanisms that can become targets for new treatment development. Two leading candidate brain regions are the hippocampus and prefrontal cortex.
Basic research has highlighted the importance of the hippocampus and surrounding medial temporal lobe for episodic memory. Interest in the medial temporal lobe was sparked by observation of marked anterograde amnesia produced by bilateral resection of this structure
(8), and subsequent studies of humans and animals with medial temporal lobe lesions
(9) documented its importance in relational binding, memory consolidation, and retrieval. Accordingly, a great deal of research has tested the hypothesis that memory deficits in schizophrenia may be due in part to medial temporal lobe dysfunction. Consistent with this hypothesis, structural and molecular hippocampal abnormalities have been documented in schizophrenia
(10,
11) . However, these patients do not exhibit the classic amnesic syndrome typical of medial temporal lobe dysfunction
(12,
13), suggesting that regions beyond this lobe may contribute to memory deficits in such patients.
Close examination of relative memory strengths and weaknesses in patients with schizophrenia reveals compelling parallels to patients with prefrontal cortex damage. Like patients with prefrontal cortex lesions
(14), individuals with schizophrenia do not spontaneously use semantic information to categorize related word lists during encoding
(13,
15) but benefit when strategies are provided
(16,
17) . Both groups also become more impaired as retrieval tasks become less structured and cognitive control demands increase
(1,
13) . These parallels, as well as evidence of structural and molecular abnormalities
(18 –
20) in the prefrontal cortex of patients with schizophrenia, contribute to the idea that a breakdown in prefrontally mediated cognitive control mechanisms may lead to episodic memory impairment in schizophrenia
(21) .
Functional imaging of episodic memory in schizophrenia has documented medial temporal lobe and prefrontal cortex dysfunction
(22), with some authors proposing a disruption in frontotemporal connectivity
(23) . However, in meta-analytic
(22) and qualitative
(23) reviews, the majority of studies reveal group differences in the prefrontal cortex and not in the medial temporal lobe
(23) . Moreover, many studies finding group medial temporal lobe differences rely on region-of-interest methods that restrict analysis to this specific region. Thus, a limitation of a previous activation likelihood estimation (ALE) meta-analysis
(22) was the combination of whole-brain and region-of-interest studies, which may have biased results in favor of selected regions of interest. ALE is a voxel-based method for finding concordance across neuroimaging studies that does not rely on author-assigned anatomical labels
(24,
25) . Our goal in this ALE study is to limit analysis to whole-brain experiments while testing the prediction that reduced activation in patients with schizophrenia during episodic encoding and retrieval is most prominent in the prefrontal cortex.
Method
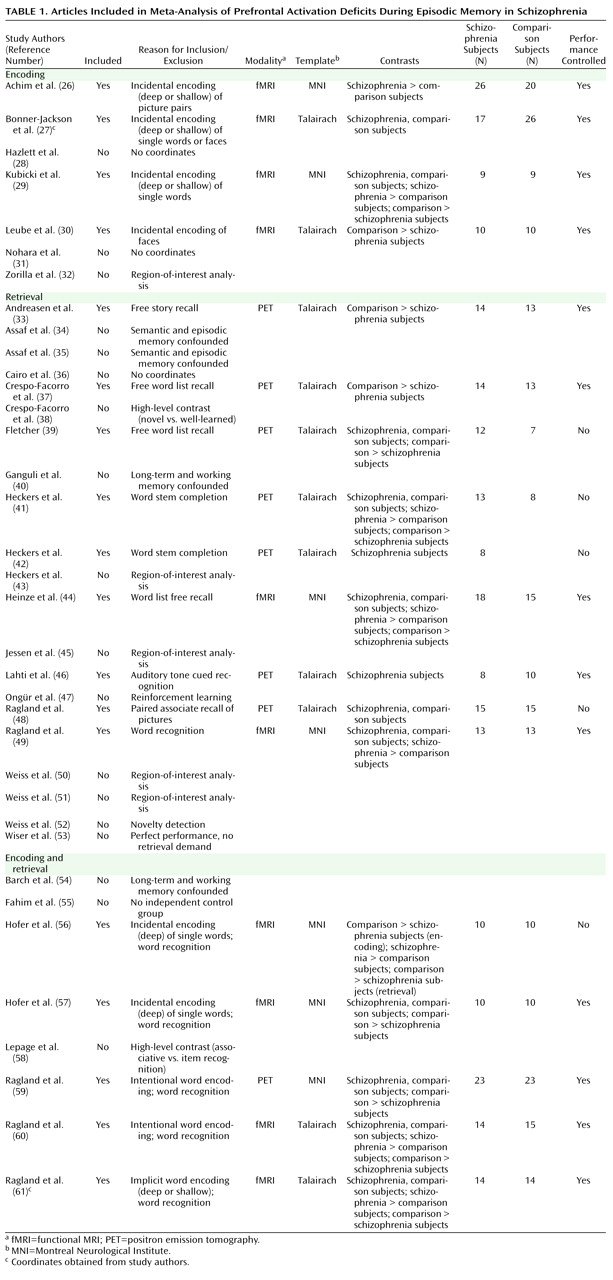
A PubMed search was conducted to identify functional MRI (fMRI) and positron emission tomography (PET) studies investigating episodic memory in patients with schizophrenia. The search identified 36 articles published through February 2008 that localized brain activity during encoding or retrieval of single stimuli (words, pictures, or tones), paired pictures, or word lists (
Table 1 ).
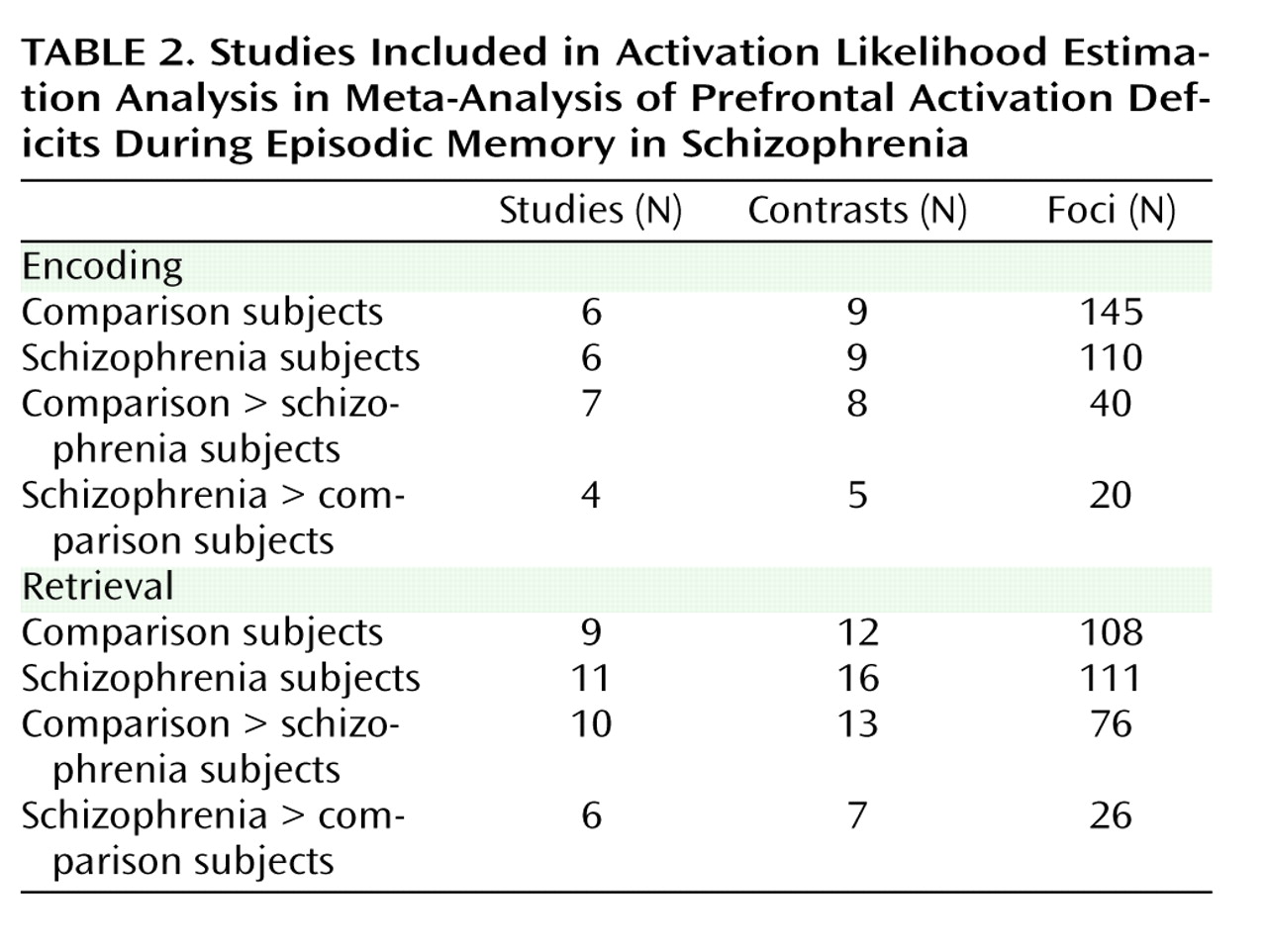
As a meta-analytic method, ALE identifies convergence across studies. However, this convergence depends on inclusion of studies that address a similar question and employ equivalent designs. The aim of the exclusions described below was to maintain a sufficient number of activation foci (
Table 2 ) while minimizing procedural differences. Accordingly, analyses were restricted to studies that employed incidental or intentional encoding tasks; examined retrieval using recognition, cued recall, or free recall tasks; and contrasted memory conditions with resting, visual fixation, or lower-level baseline tasks (e.g., word reading).
ALE produces convergence maps of brain activation by examining the probability that spatially smoothed activation foci from individual studies occur across multiple studies. This approach tests the null hypothesis that the location of activated foci is equal at every voxel against an alternative hypothesis that activated foci are spatially distributed. This null assumption is violated by region-of-interest studies because they do not provide results for all voxels in the brain. The need for studies to examine the whole brain eliminated five studies
(32,
43,
45,
50,
51) . ALE also requires reporting of results in Talairach or Montreal Neurological Institute (MNI) coordinates, a requirement that eliminated three additional studies
(28,
31,
36) . Four studies reported results only for higher-level contrasts (e.g., associative versus item encoding), and authors were contacted and asked whether they could provide lower-level baseline results. The authors of two studies
(27,
61) provided additional results, which were included in our analysis; the remaining two studies
(38,
58) were excluded. Four encoding studies
(34,
35,
40,
54) were omitted because memory was confounded with either semantic or working memory tasks in the same contrast, and two retrieval studies were omitted because they used measures of either reinforcement learning
(47) or novelty detection
(52) . An additional retrieval study was omitted because of absence of retrieval demands, as subjects had been practiced to perfect task performance
(53) . Finally, one study was excluded because it lacked an independent comparison group
(55) .
With these selection criteria, a sample of 18 articles was established (
Table 1 ). In two studies
(33,
37) patients were unmedicated at the time of testing. Coordinate (x, y, z) results from each study were divided into groups based on stage of memory processing: encoding (eight studies) and retrieval (14 studies). Encoding instructions were either intentional (e.g., “remember these items”) or incidental (deep versus shallow encoding). Incidental instructions were to make semantic discriminations, such as “abstract/concrete” during deep encoding, and perceptual discriminations, such as “uppercase/lowercase” during shallow encoding. Retrieval was tested with either “old”/“new” recognition tasks, cued recall (word stem completion), or free recall. Unlike in the previous ALE meta-analysis
(22), retrieval was not subdivided into high versus low performance because of an insufficient number of foci once region-of-interest studies were excluded. The number of foci was sufficient to perform a separate ALE analysis of incidental tasks in which an encoding strategy was provided. We also repeated between-group ALE analyses after excluding studies that did not control for group performance differences (
Table 1 ).
Encoding and retrieval contrasts were examined separately for within-group activations in schizophrenia patients and healthy comparison subjects, and for between-group comparisons (comparison subjects > schizophrenia patients and schizophrenia patients > comparison subjects). Coordinates using the MNI template were spatially renormalized to Talairach space using the Lancaster transform
(62) . MNI coordinates in one study
(44) had been converted to Talairach space using the Brett (mni2tal) transform
(63) ; we therefore uncorrected and reconverted them using the Lancaster transform, which better reduces disparity between Talairach and MNI coordinates
(62) .
ALE meta-analyses were performed on the contrasts specified in
Table 2 using a full width at half maximum of 12 mm
(24) in GingerALE 1.2, distributed by the BrainMap project
(64,
65) . Statistical significance was determined within GingerALE 1.2 using a permutation test of 5,000 permutations. ALE images were thresholded at significance level of 0.05, false-discovery-rate-corrected for multiple comparisons
(66), with a cluster extent threshold of 300 mm.
Discussion
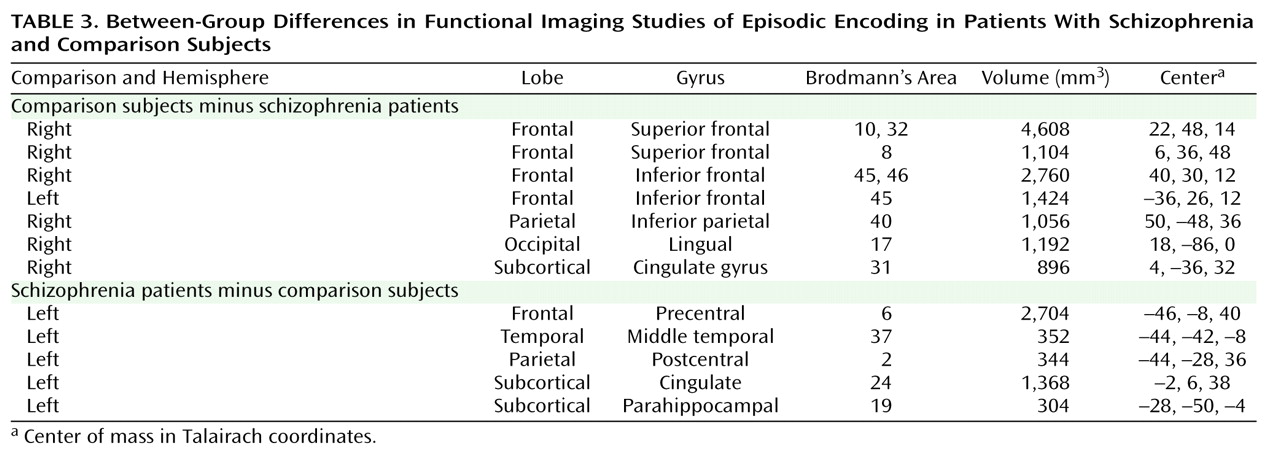
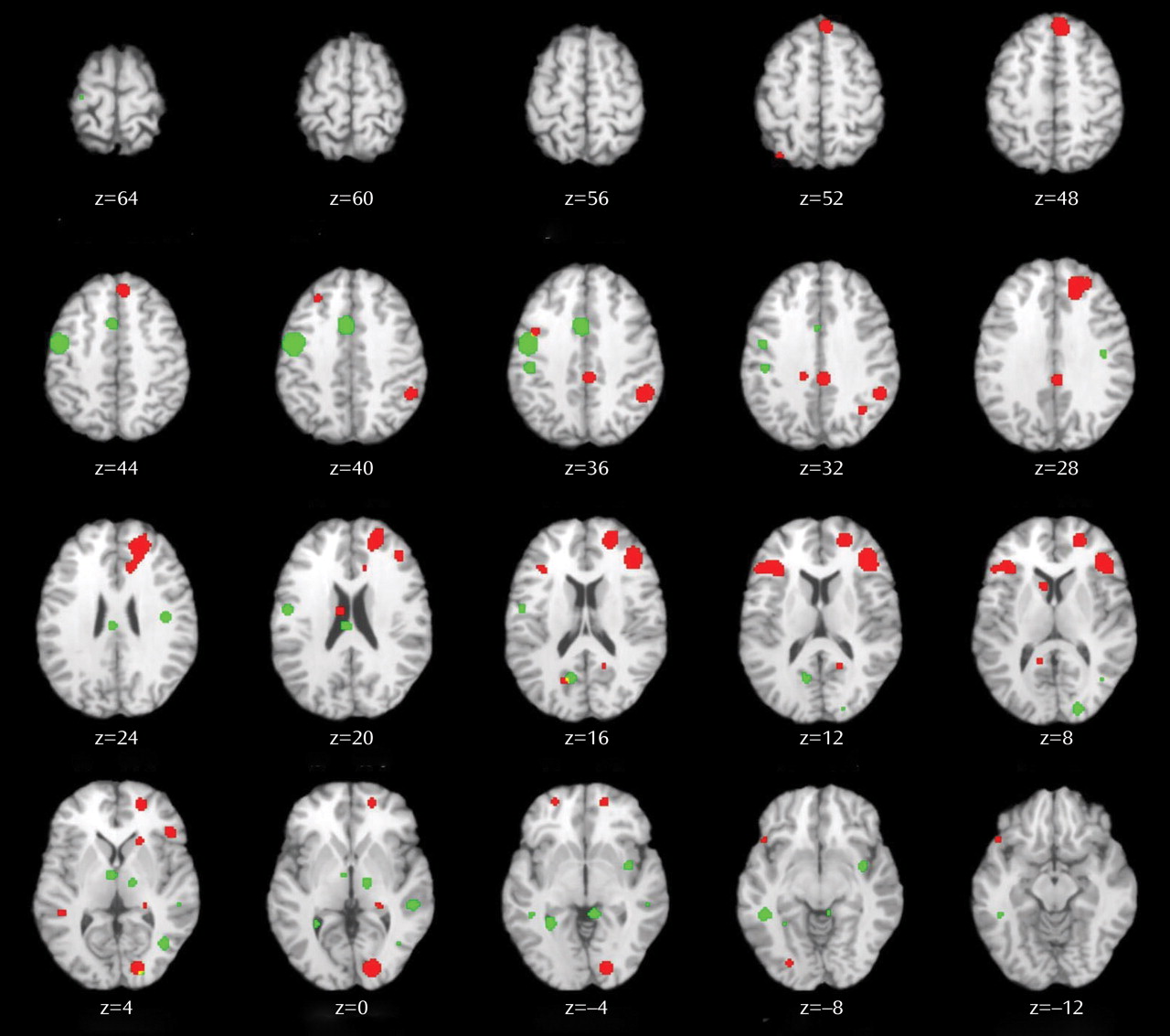
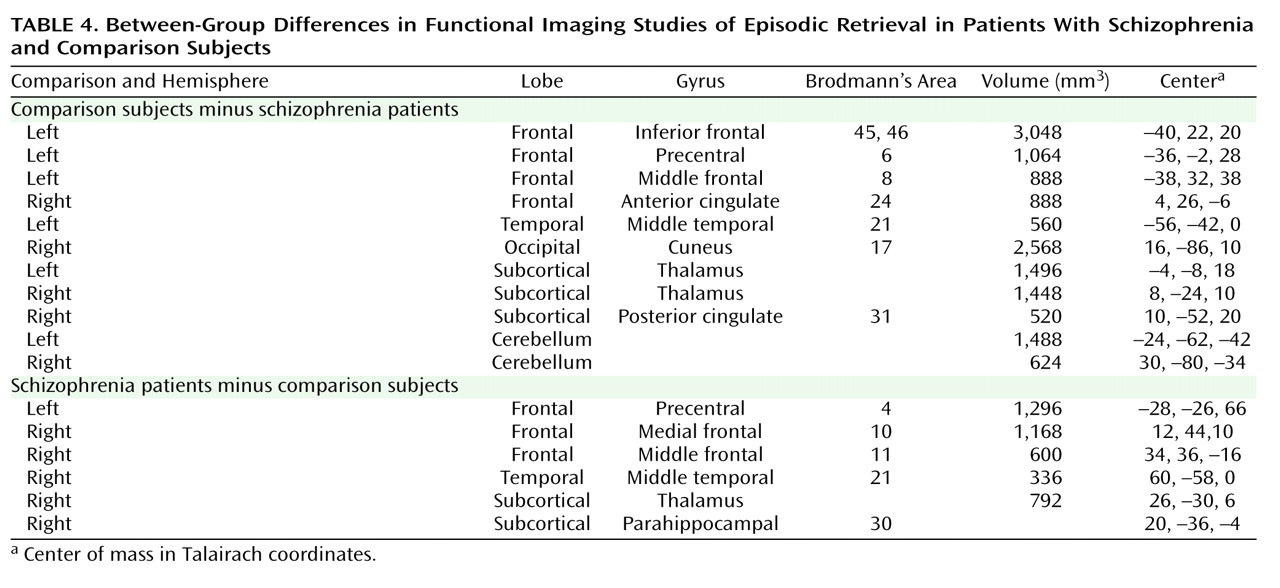
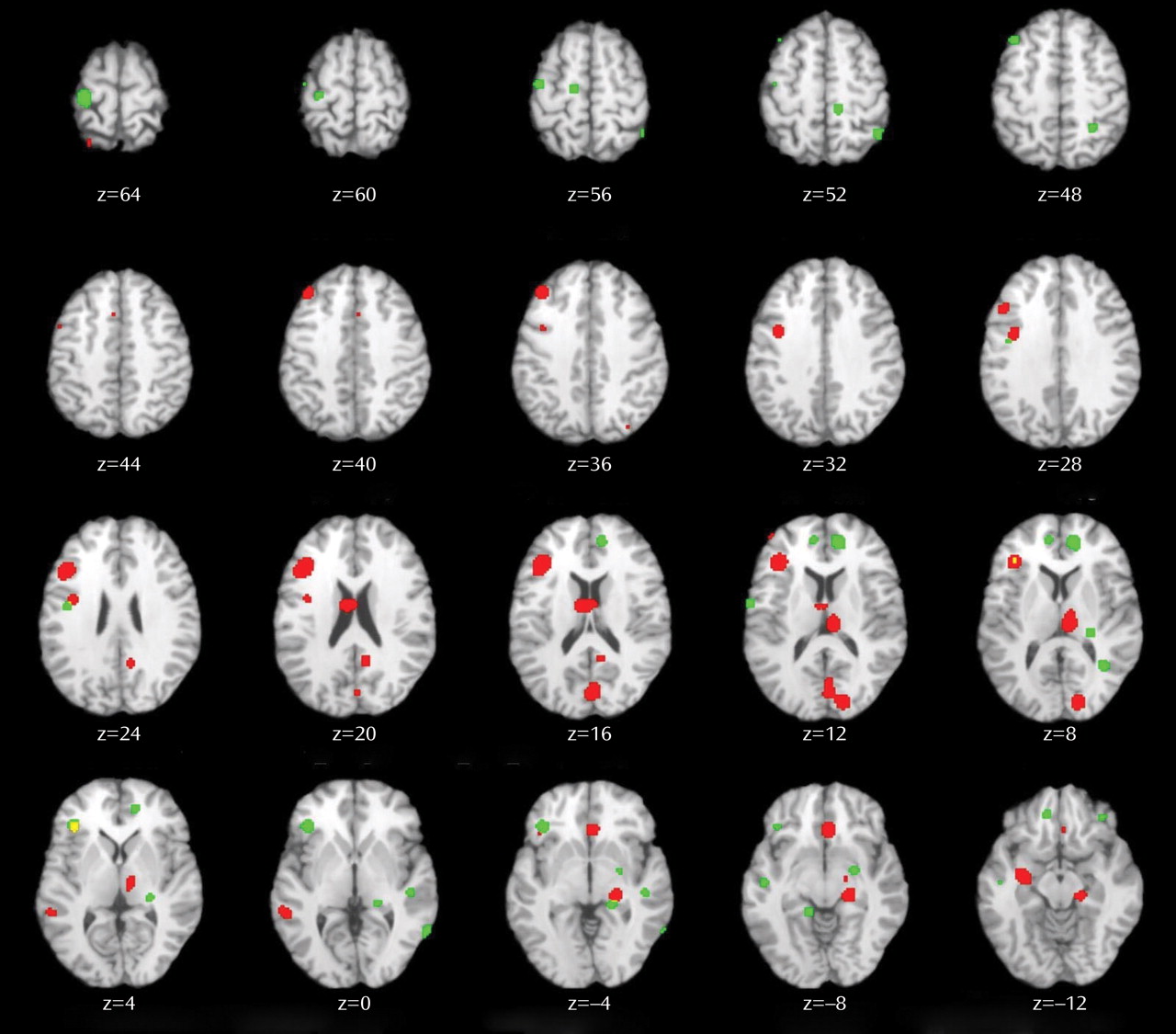
This quantitative meta-analysis of episodic memory functional imaging studies, with a combined sample of 123 patients with schizophrenia and 137 healthy comparison subjects, found prominent deficits in the prefrontal cortex in patients. During encoding, these deficits were in the left frontopolar (BA 10, 32), ventrolateral (BA 45), and dorsolateral (BA 46) prefrontal cortex. During retrieval, the largest effects were also in the left ventrolateral and dorsolateral prefrontal cortex. When patients were provided with semantic encoding strategies, dorsolateral prefrontal cortex deficits remained, but activation of the ventrolateral prefrontal cortex was no longer reduced. This ventrolateral prefrontal cortex sparing is consistent with previous univariate
(27,
61,
67 –
70) and meta-analytic studies
(71), which suggests that the ventrolateral prefrontal cortex may compensate for reduced dorsolateral prefrontal cortex function during working memory and episodic encoding
(21,
72,
73) . In contrast, there was no evidence of reduced hippocampal or surrounding medial temporal lobe activation in patients versus comparison subjects during encoding or retrieval. The only group difference in the medial temporal lobe was a relative
increase in activation in patients in the parahippocampal gyrus during encoding and retrieval. This prominent prefrontal dysfunction was not secondary to unequal performance, as prefrontal cortex deficits remained when studies that did not control for group performance differences were eliminated.
Patient dysfunction during encoding was relatively circumscribed, including portions of the prefrontal cortex and a default mode network
(74) previously associated with increased task-related deactivation in schizophrenia across a wide array of behavioral paradigms
(75,
76) . Frontal lobe dysfunction was localized to the frontopolar, ventrolateral, and dorsolateral prefrontal cortex. These three regions are associated with discrete working memory and episodic encoding functions. The frontopolar prefrontal cortex provides for selection and processing of subgoals during working memory
(77,
78) ; the ventrolateral prefrontal cortex is involved with semantic processing and working memory maintenance
(79) and binding of items with their context during working memory and episodic encoding
(80) ; and the dorsolateral prefrontal cortex is involved with active working memory maintenance and manipulation
(67,
81) and with processing relationships between items during encoding
(82,
83) . These functional deficits suggest that patients have difficulty selecting and maintaining rules to process items in their context and in relation to each other to facilitate encoding. If rules are provided, schizophrenia patients appear capable of ventrolateral prefrontal cortex-mediated item-specific processing but remain unable to recruit the dorsolateral prefrontal cortex to establish more interactive relational memory representations, leading to severe deficits in relational memory
(21) .
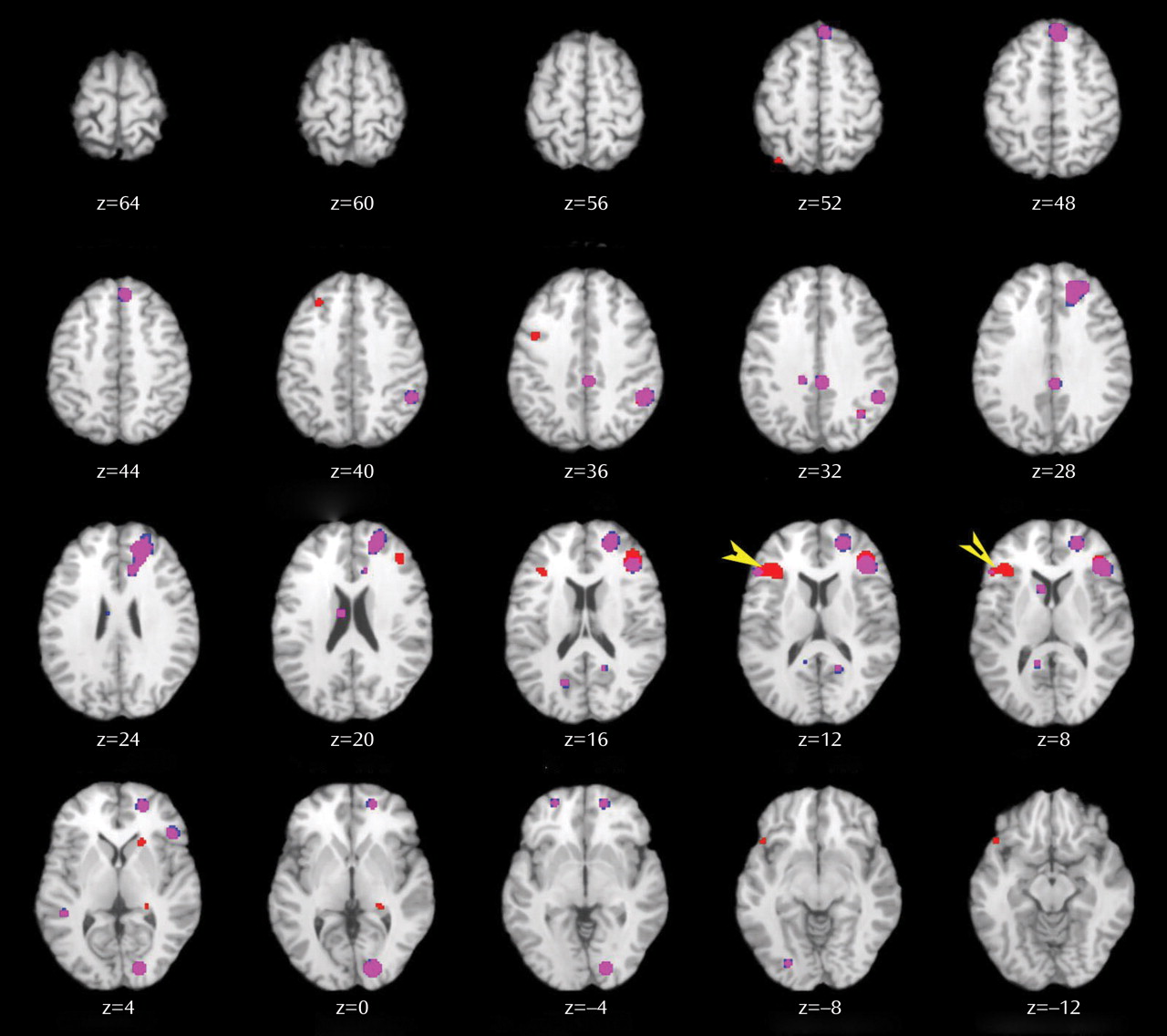
Patient deficits were more distributed during retrieval, even after group performance differences were eliminated. Impairments were noted in a frontocortical-cerebellar-thalamic network previously described by Andreasen and colleagues
(33,
37) as creating a condition of “cognitive dysmetria” in which patients have trouble coordinating sensorimotor and mental processes. However, cognitive dysmetria was formulated to extend beyond episodic retrieval, and our finding that these distributed regions were not impaired during encoding suggests that cognitive dysmetria cannot uniquely explain the pattern of our findings. On the other hand, evidence is accumulating that many components of this distributed network mediate specific cognitive functions that are important for successful episodic retrieval. The dorsolateral prefrontal cortex is associated with postretrieval monitoring
(84,
85), the anterior cingulate gyrus with error or conflict detection
(86), the thalamus with attention and working memory
(87), and the cerebellum with working memory and mental flexibility
(88) . These combined functional deficits suggest a scenario in which schizophrenia patients have difficulty monitoring their response output and detecting errors in order to flexibly adjust signal-detection thresholds to optimize sensitivity to targets while avoiding nontargets.
The aforementioned regions serve functions beyond episodic memory and are impaired in schizophrenia during other cognitive and emotional paradigms. The dorsolateral prefrontal cortex is broadly implicated in cognitive control mechanisms that allow information processing and behavior to vary adaptively from moment to moment, depending on current goals
(89) . Our ALE findings may reflect a more general deficit in control mechanisms such as context maintenance
(90) . Likewise, the thalamus is a central relay station that gates and filters sensory input to the cortex
(91), and thalamic dysfunction may reflect a fundamental deficit in sensory integration. Because ALE combines disparate studies, our analysis is not sufficiently constrained to establish functional specificity of these memory deficits. Establishing this level of specificity would require a focused effort, such as the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative
(92), to translate cognitive neuroscience tasks that are designed to parse these complex and overlapping functions to the study of schizophrenia.
The only group difference in the medial temporal lobe was increased activation in the parahippocampal gyrus in patients during encoding and retrieval. Overactivation in this and other regions (sensorimotor, middle cingulate gyrus, and middle temporal gyrus) in patients may reflect inefficient, compensatory brain activity as extraneous task-related activation can also be seen at early stages of learning before an optimal cognitive strategy has been reached
(93), and during reorganization following acute brain injury
(94) . The parahippocampal gyrus is also associated with familiarity-based episodic retrieval
(9), and excess activity in this region may reflect patients’ overreliance on familiarity-based retrieval because of a specific recollection deficit
(95) . Unlike the previous ALE study
(22), we did not find reduced hippocampal activation in schizophrenia, which may have resulted from our exclusion of region-of-interest studies to preserve the validity of the ALE method. The absence of hippocampal findings on the whole-brain level may have reflected increased susceptibility of small regions to smoothing artifact, although there were still no differences when the smoothing kernel was reduced to 6 mm and the ALEs repeated (data available on request). The absence of hippocampal findings may also reflect minimal relational binding demands
(96,
97), as most included studies employed overlearned word stimuli. As more studies are performed that contrast relational versus item-specific encoding and retrieval, it will be interesting to see if medial temporal lobe differences can be detected on a meta-analytic level.
Several caveats must be considered when interpreting the results of this meta-analysis. First, in light of the limited number of articles meeting the study criteria, it was necessary to combine studies with disparate encoding and retrieval conditions and varying stimulus characteristics. This has the advantage of revealing the most robust and replicable task effects and group differences across memory paradigms, but the disadvantage of limiting our ability to ascribe specific brain regions to discrete memory processes. With a larger group of studies to choose from, it would be informative to segregate ALE analyses based on encoding condition (e.g., item-specific versus relational), stimulus modality (e.g., verbal, nonverbal), and retrieval task (e.g., recall versus recognition, cued versus uncued, item-specific versus relational). Second, the ALE method does not account for differences in sample size across included studies. This can be a strength in that it reveals the central tendency of the data, but a limitation if a study with a small sample and large effects that may not replicate is included and unduly influences overall results. Fortunately, the majority of studies had relatively equal sample sizes. Finally, all but two studies examined patients while they were receiving antipsychotic medication, which raises the question of drug effects. However, a qualitative examination of the two studies of unmedicated patients, both retrieval studies
(33,
37), revealed the same pattern of reduced patient activation in prefrontal, cingulate, thalamic, and cerebellar regions that was seen in the studies of medicated patients. This apparent lack of medication effects is consistent with several studies of unmedicated patients
(54,
69) that documented reduced prefrontal activation in patients that was not restored by antipsychotic treatment
(98) .
In sum, the results of this study provide strong support for the conclusion that episodic memory impairments in schizophrenia during encoding and retrieval are related to a reduction in memory control mechanisms implemented by the anterior, ventrolateral, and dorsolateral prefrontal cortex. These results suggest that behavioral interventions developed for remediating memory deficits in patients with frontal lobe damage
(99) may also be applicable to schizophrenia. Use of pharmaco-fMRI
(100,
101) to identify compounds that improve prefrontal function
(102) may also lead to new medications that improve memory and daily functioning in individuals with schizophrenia.