Schizophrenia is commonly treated with a range of medications that raise serum anticholinergic activity
(1 –
3) . Preclinically, these medications are known to affect nicotinic and muscarinic receptors
(4) . Clinically, medication-related muscarinic anticholinergic effects adversely affect cognitive performance, especially on measures of learning and memory, in both elderly persons and patients with schizophrenia
(1,
2,
5 –
11) . This finding is consistent with a wealth of basic science research demonstrating that cholinergic projections from the basal forebrain throughout the cerebral cortex play a key role in attention and memory
(12 –
17) .
Given that we now know it is imperative to treat the cognitive dysfunction of schizophrenia, and given that deficits in attention and memory are critically related to functional outcome, it becomes especially important to understand the role of medication-induced anticholinergic effects in both baseline cognitive performance and in response to treatment interventions. For example, emerging work in basic neuroscience indicates that cholinergic projections play an active role in cortical neuroplasticity by optimizing the detection of relevant signals during both bottom-up and top-down information processing
(13,
14,
17 –
20) . These data suggest that the use of medications with muscarinic-blocking effects may have serious consequences for any cognitive treatment that attempts to harness mechanisms of cortical plasticity, particularly in view of the apparently synergistic interactions between muscarinic and nicotinic systems in the optimal functioning of attention and working memory processes in humans
(16,
21) .
1. What is the association between medication-induced anticholinergic effects and baseline neurocognitive performance in a well-characterized sample of patients with chronic schizophrenia? Would we replicate earlier findings of a negative association
(1,
2,
5,
8 –
11), using a more direct method of assessing anticholinergicity, a larger sample, and a comprehensive cognitive battery based on the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative?
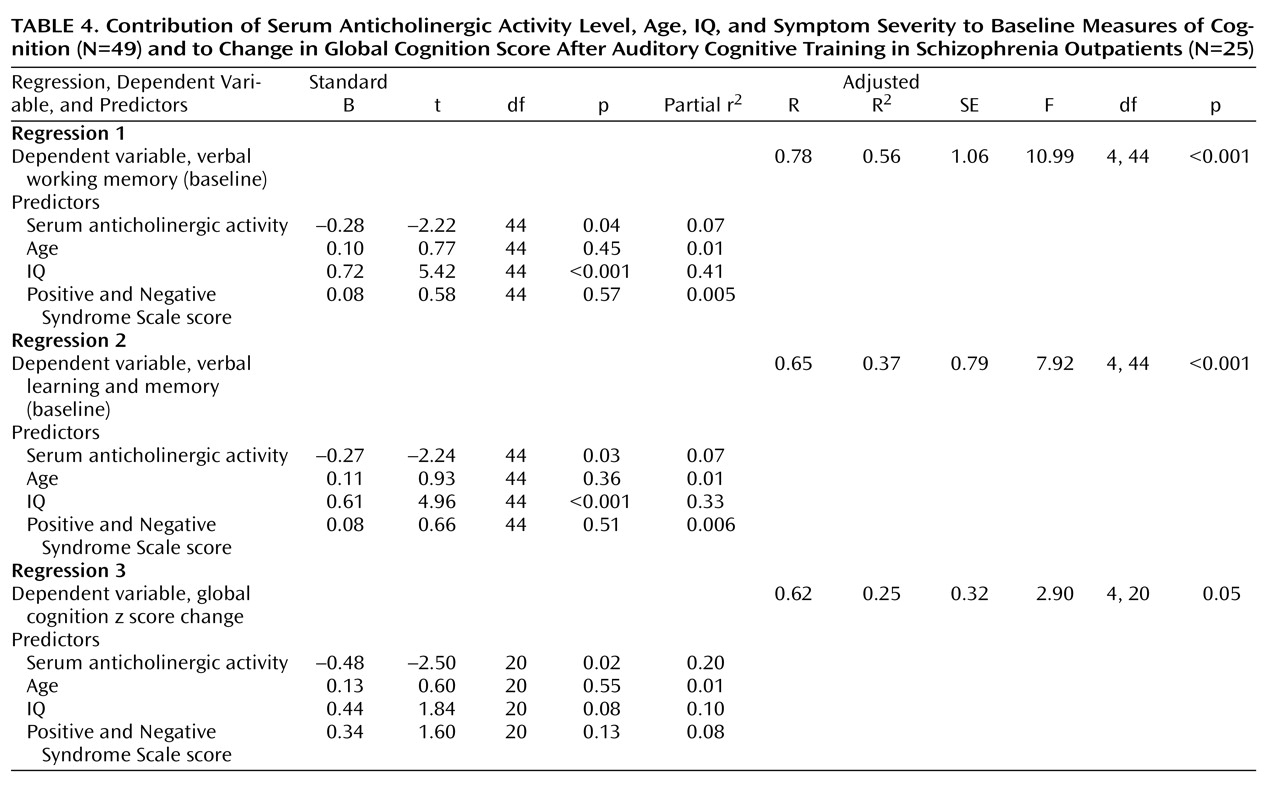
2. What portion of the variance in baseline cognition is uniquely accounted for by anticholinergic burden in a clinically stable sample of schizophrenia patients, after age, IQ, and symptom severity have been controlled for?
3. What is the relationship between anticholinergic burden and the response to intensive “neuroplasticity-based” auditory training (i.e., to cognitive change)? What portion of the response to this cognitive training is uniquely accounted for by serum anticholinergic activity after age, IQ, and symptom severity have been controlled for?
Method
Participants
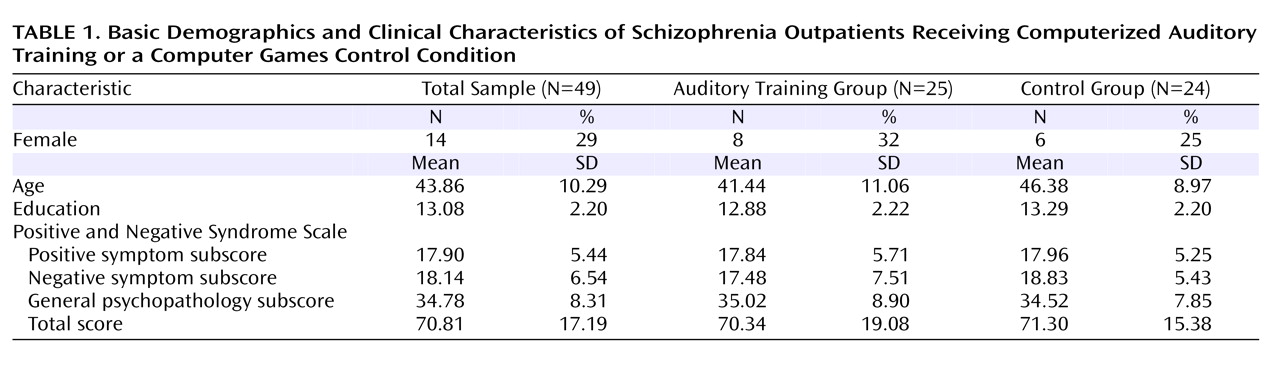
Fifty-five clinically stable, chronically ill schizophrenia outpatients 20–59 years of age were recruited from community mental health centers to undergo a randomized clinical trial of a computerized neuroplasticity-based auditory training program. After the study procedures were explained, participants gave written informed consent and underwent baseline clinical and cognitive assessments over a 2–3 week period, including blood draws for serum anticholinergic activity. Participants were then stratified by age, gender, education, and symptom severity and randomly assigned to either the auditory training condition or a control condition of commercial computer games. Because four patients refused blood draws and blood results for two patients were lost to laboratory error, serum anticholinergic levels were available for 49 participants.
Table 1 summarizes the basic demographic characteristics of the sample.
Participants engaged in either auditory training (N=25) or a computer games control condition (N=24) for 1 hour a day, 5 days a week, for an average of 10 weeks (verified by training software). All participants received nominal payment for participation. Participants remained on stable doses of medications during the study, defined as no dose change >10%, as prescribed by their community psychiatrists. Participants’ medication regimens varied widely, ranging from monotherapy with a second-generation antipsychotic to several antipsychotics plus other psychotropic medications.
Clinical and Neurocognitive Assessments
The Positive and Negative Syndrome Scale (PANSS)
(23) and all MATRICS-recommended measures
(24), with the exception of the mazes subtest of the Neuropsychological Assessment Battery, were administered at baseline and after the intervention. The Tower of London subtest of the Brief Assessment of Cognition in Schizophrenia
(25) was substituted for the mazes subtest to assess problem-solving. Because of software difficulties, data from the Continuous Performance Test–Identical Pairs Version were not interpretable. At the time this study was initiated, the MATRICS Consensus Cognitive Battery (MCCB) was not yet available, but the list of recommended measures for the MCCB beta version was available on the MATRICS web site. We obtained the MATRICS-recommended measures from test publishers and converted raw scores to z scores using normative data, stratified by age, published by test authors. All measures were distinct and independent from tasks practiced during the auditory training, and assessment personnel were blind to group assignment. Alternate forms of tests were administered and counterbalanced at baseline and after the intervention for tests sensitive to practice effects.
Neuroplasticity-Based Computerized Auditory Training
Training of auditory/verbal processing was provided by software developed by Posit Science, Inc. In these computerized exercises (described in detail in reference 22), participants were driven to make progressively more accurate distinctions about the spectrotemporal fine structure of auditory stimuli and speech under conditions of increasing working memory load, and to incorporate and generalize their improvements in auditory signal salience into working memory rehearsal and verbal learning. The exercises were continuously adaptive: they first established the precise parameters within each stimulus set required for the participant to maintain 80% correct performance; once that threshold was determined, task difficulty increased systematically and parametrically as performance improved. In all exercises, correct performance was heavily rewarded in a game-like fashion through novel and amusing visual and auditory embellishments as well as the accumulation of points.
Serum Anticholinergic Activity
During baseline testing, two blood draws were taken from all patients 1 week apart and averaged to enhance the reliability of the measure. In addition, 13 randomly selected participants had blood draws again 10 weeks later to determine the correlation of levels at study entry with levels drawn after the intervention. This correlation was highly significant (Pearson’s r=0.89, p<0.003), indicating that levels remained constant throughout the intervention period in our participants.
Serum anticholinergic activity
(25) was measured using the method of Tune and Coyle
(7,
26 –
28) . The method is a competitive radioreceptor binding assay using prepared muscarinic membranes from rat forebrain (cerebral cortex and striatum, Sprague-Dawley males) and 3-hour quinuclidinyl benzilate (QNB) as a radioligand with various concentrations of atropine (Sigma-Aldrich, St. Louis, Mo.) used in a standard curve for displacement. Patient serum is added and incubated for 1 hour at 22°C. Ice-cold phosphate buffer is added and the ligand-receptor complex is isolated by aspiration over Whatman GF/B filters (Piscataway, N.J.) using a Brandel Cell Harvester (Gaithersburg, Md.). Filters are added to vials with scintillation cocktail and counted using a Beckman Liquid Scintillation Counter (Fullerton, Calif.). This assay measures the free drug in sera that binds to the muscarinic receptors. Anticholinergic activity is reported in picomoles of atropine equivalents per milliliter (pmol/ml), based on the amount of 3-hour QNB displacement that would have been caused by a standard amount of atropine in a 200-μl sample. The results are then normalized to 1 ml.
Serum anticholinergic activity is thought to reflect the cumulative muscarinic anticholinergic effect of all exogenous substances the person has taken (prescribed and over-the-counter medications and supplements) and their metabolites. The limit of detection for this assay is 0.25 pmol/ml, with a standard linear curve (r=0.99) from 0.50 to 25.00 pmol/ml and good interassay and intra-assay reproducibility for these concentrations (coefficient of variations <12%).
Data Analysis
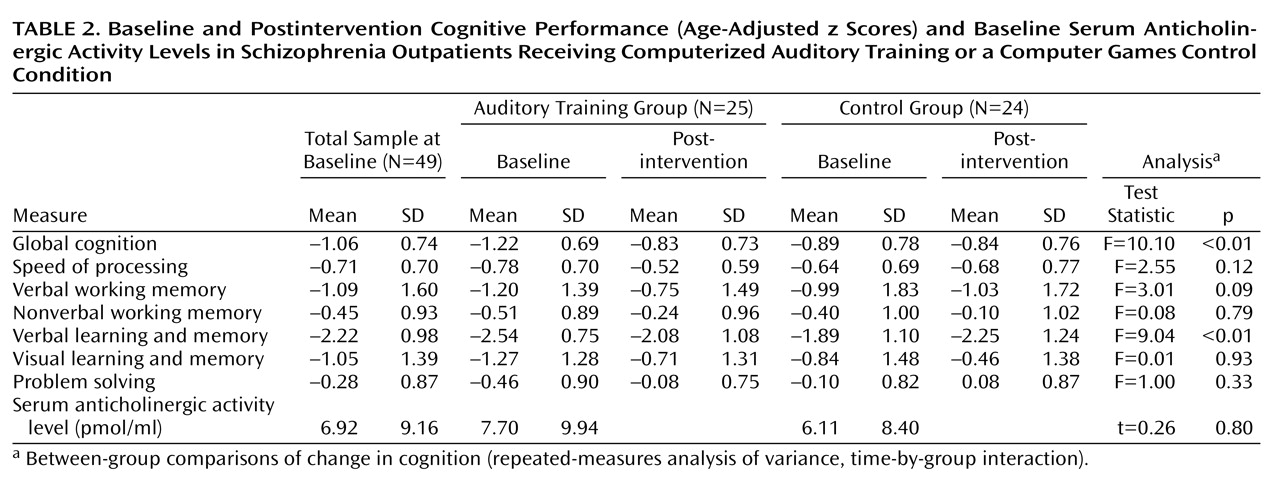
The distributions of all variables were evaluated for normality and screened for outliers. The baseline serum anticholinergic activity distribution was skewed (consistent with other studies [
6,
7 ]) and was normalized using square-root transformation. All neurocognitive variables were normally distributed after winsorizing of outlying values. Measures of cognitive performance were computed for six MATRICS-defined cognitive domains—speed of processing, verbal working memory, nonverbal working memory, verbal learning and memory, visual learning and memory, and problem solving—and a composite score of global cognition was computed as the average of all measures. Repeated-measures analysis of variance (ANOVA) was used to test group differences in cognitive performance from baseline to postintervention assessments. Pearson correlations were computed to determine the association between serum anticholinergicity and baseline cognitive performance in the total sample and between anticholinergicity and response to treatment in the auditory training group, defined as change in the cognitive measures (postintervention minus baseline performance). Multiple regression analyses were used to test the contributions of serum anticholinergic activity, age, IQ, and symptom severity to baseline cognitive measures in the total sample and to response to treatment, defined as z score change in the global cognition composite score, in the auditory training group. Multivariate ANOVA was used to test the difference between participants from the highest and lowest quartiles of serum anticholinergicity in the multivariate effect and main effects of serum anticholinergic activity, response to treatment (defined as z score change in global cognition), and PANSS total score.
Discussion
We examined serum anticholinergic activity, determined via radioreceptor binding assay, and neurocognitive performance, assessed via the MATRICS battery, in a moderately sized sample of clinically stable adult schizophrenia outpatients receiving community-based medication treatment. Only a small minority of patients (N=3) of this sample of 49 patients had no detectable anticholinergic activity, while over half had levels ≥3 pmol/ml. To place this in a meaningful clinical and cognitive context, Mulsant et al.
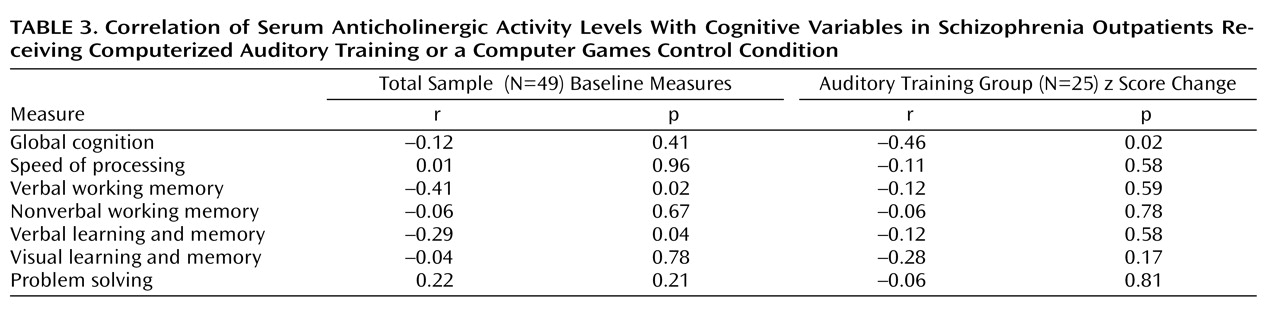
(7) found that healthy community-dwelling older adults with anticholinergic activity levels greater than 2.8 pmol/ml were 13 times more likely than those with undetectable levels to have a score ≤24 on the Mini-Mental State Examination. Similarly, we found a significant relationship between anticholinergic burden and baseline performance in working memory and in verbal learning and memory in these schizophrenia patients, with serum anticholinergicity accounting for 7% of the variance in these crucial cognitive functions, independent of IQ, age, and symptom severity.
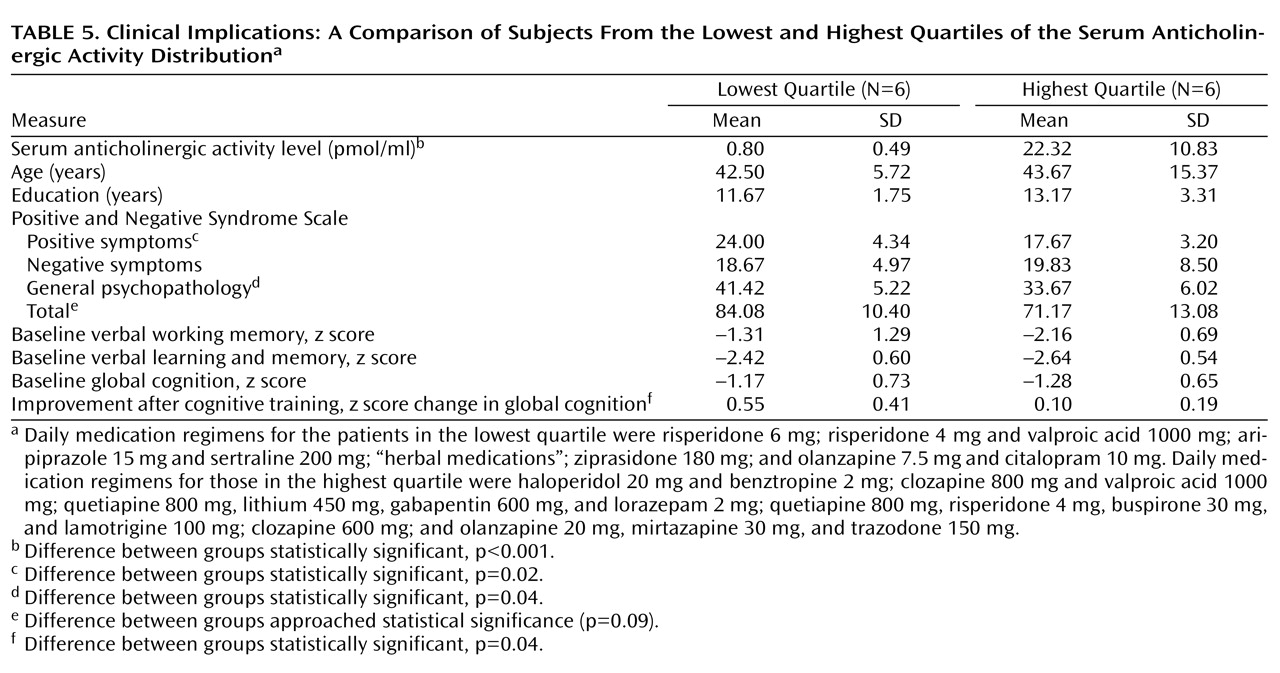
These results were obtained in patients who volunteered to participate in a demanding and time-intensive research study, and who thus were treatment responsive, compliant, and relatively higher-functioning. As indicated by our quartile data in
Table 5, one might anticipate that anticholinergic burden would be greater in patients identified as “treatment resistant” and treated with clozapine and/or multiple medications at higher doses
(28) . Although any generalizations from our study must be undertaken with caution, our results suggest that clinically significant medication-induced anticholinergic activity may be relatively common in the community treatment of schizophrenia. Furthermore, it has meaningful deleterious consequences on cognitive function in patients, suggesting a need for caution when prescribing medications with known anticholinergic effects, such as clozapine, olanzapine, quetiapine, benztropine, and certain first-generation agents. It is likely that anticholinergic burden exacerbates the underlying neurocognitive pathology of schizophrenia, similar to what has been seen with cerebrovascular disease in the elderly
(29) .
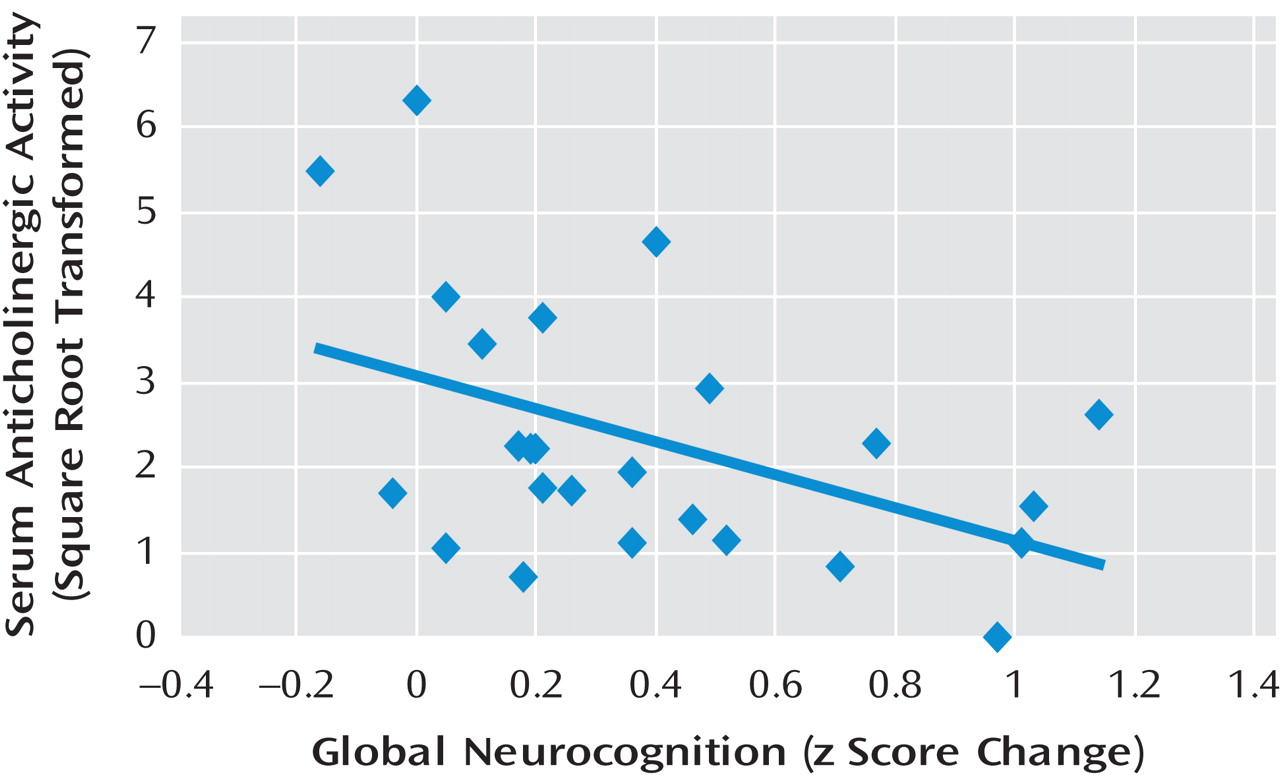
Of perhaps even greater concern is our finding that anticholinergic burden is significantly associated with a lowered response to the effects of intensive “neuroplasticity-based” computerized cognitive training. In our cognitive training group, serum anticholinergic activity accounted for 20% of the variance in the global cognition change score—significantly more than IQ, age, or symptom severity. The relative contribution of anticholinergic activity and of IQ to measures of baseline cognition differed from the relative contribution of these variables to cognitive change, with anticholinergicity playing a greater role in the latter measure. Serum anticholinergic activity was negatively associated with verbal working memory and long-term memory at baseline, but with global cognition
change after training. This pattern of results is consistent with emerging research indicating that baseline variables that show significant cross-sectional associations with outcome measures in schizophrenia are not always identical to the variables that are associated with change in outcome
(30,
31) . Anticholinergic burden may be an important mediator, with effects on specific cognitive domains when measured at a single time point, but with even larger effects on cognitive change resulting from training. Interestingly, patients in the lowest anticholinergicity quartile, who were on more conservative medication regimens, had
higher ratings on positive symptoms but also showed a greater response to cognitive training compared to patients in the upper quartile. We speculate that a conservative approach to the pharmacologic treatment of schizophrenia, combined with behavioral techniques for managing low-level psychotic symptoms, might optimize patients’ ability to benefit from cognitive interventions.
At this point, we cannot say whether the negative relationship between anticholinergic activity and cognitive outcome is unique to our particular neuroplasticity-based computerized cognitive training approach or whether this finding would also be observed using other remediation methods. As far as we know, no other study has investigated the association between anticholinergic activity in schizophrenia and the response to a behavioral treatment. These results become especially germane in light of the growing awareness that cognitive function influences the ability of people with schizophrenia to benefit from psychosocial interventions
(32 –
34) . It appears that if we wish to maximize patients’ response to rehabilitation, we must take care to minimize their anticholinergic burden.
There were several limitations to this study. The sample was of modest size, and it was not representative of the schizophrenia population as a whole. Because we did not obtain serial anticholinergic activity measures on all participants in the auditory training group throughout the course of the 10 weeks, we cannot be certain that the measures obtained at study entry remained consistent throughout the duration of the intervention; however, the highly significant correlation we found between levels at study entry and 10 weeks later in 13 randomly selected participants strongly suggests that this was the case.
Finally, because of technical difficulties, we were unable to obtain reliable data on the Continuous Performance Test–Identical Pairs Version, a computerized attentional measure, and therefore do not know whether anticholinergic activity would show a significant negative association with this task, either at baseline or after training. Harvey et al.
(35) found that patients being treated with risperidone showed practice-related improvements in visual performance on the Continuous Performance Test, but patients being treated with conventional antipsychotic agents did not.
Although it is well established that older adults are highly susceptible to cognitive impairment and delirium as a result of anticholinergic burden, this study demonstrates that effects are also detectable in younger individuals with schizophrenia, are quantifiable, and are therapeutically relevant. For clinical investigators, these effects deserve further research in other behavioral and perhaps pharmacologic paradigms; if we reduce anticholinergic load, we may foster patients’ ability to benefit from cognitive enhancement methods by maximizing their verbal memory functions. For the treating clinician, the “memory” implications are simpler: we would all do well to remember the cognitive cost of medications that carry a high anticholinergic burden.