Auditory verbal hallucinations, or 'hearing voices,' constitute a cardinal feature of psychosis. The pathophysiology of auditory hallucinations remains unknown. Previous research has shown that these hallucinations in schizophrenia are responsive to antipsychotic medication in approximately 70% of patients (
1) and that the antipsychotic effect of these agents is most likely mediated by antagonism at the dopamine type 2 (D
2) receptors (
2). Therefore, a dopaminergic component is presumed to be involved in the origin of auditory hallucinations.
A second line of evidence is derived from neuroimaging studies demonstrating consistent activation of bilateral language-related areas during auditory hallucinations (
3–
9), with the most prominent activation in the right homologue of Broca"s area (
9). However, since these language-related areas barely have any D
2 innervations (
10,
11), this cannot easily be linked to the suspected dopaminergic component. Moreover, although functional imaging of activity during hallucinations is helpful in understanding which regions are involved in the experience of hearing voices, it cannot explain how and where these experiences originate in the brain. Because auditory hallucinations arise without an external source (i.e., the experience of an actual voice), they must be triggered internally. Studying brain activation in the time period preceding the hallucinations might reveal this trigger. Therefore, we not only investigated brain activation during auditory hallucinations but also identified brain activation prior to these hallucinations.
Results
Clinical Evaluation
Patients were chronically psychotic, with a mean total PANSS score of 73 (SD=15) for the 24 patients in the analysis of brain activation during hallucinations (analysis 1). These patients had an average score of 19 (SD=5) on the positive subscale, an average score of 19 (SD=0.5) on the negative subscale, and an average score of 35 (SD=9) on the scale assessing general psychopathology. The 15 patients in the analysis of brain activation prior to hallucinations (analysis 2) had a mean total PANSS score of 73 (SD=18). Their average scores on the positive, negative, and general psychopathology subscales were 20 (SD=5), 19 (SD=6), and 35 (SD=10), respectively. Details about the hallucinations as rated using the Psychotic Symptom Rating Scales interview are listed in
Table 2, in which a rating of '1' signifies absent hallucinations or a very mild form of hallucinations during the last 3 months.
Hallucinations During fMRI Scans
The average number of hallucinations during the fMRI scans was 14 (SD=0.85) for patients in the first analysis. The average duration of a hallucination was 17 seconds (SD=0.28), adding up to a mean total duration of hallucinations of 151 seconds (SD=121).
For the second analysis, the average number of hallucinations, average duration of a hallucination, and mean total duration of hallucinations were 11 (SD=5), 16 seconds (SD=15), and 129 seconds (SD=116), respectively. The average time between successive hallucinations was 38 seconds (SD=29).
In the comparison group, the average number of balloon squeezes was 12 (SD=4). The average duration of a balloon squeeze was 10 seconds (SD=6), adding up to a mean total duration of balloon squeezes of 122 seconds (SD=75). The average duration between successive balloon squeezes was 33 seconds (SD=20).
No statistical differences were found between the patient group in the second analysis and the comparison group for the mean number, mean duration, and total duration of the hallucinations/balloon squeezes and for the time between successive squeezes and releases.
fMRI
Analysis 1: Brain activation during auditory verbal hallucinations
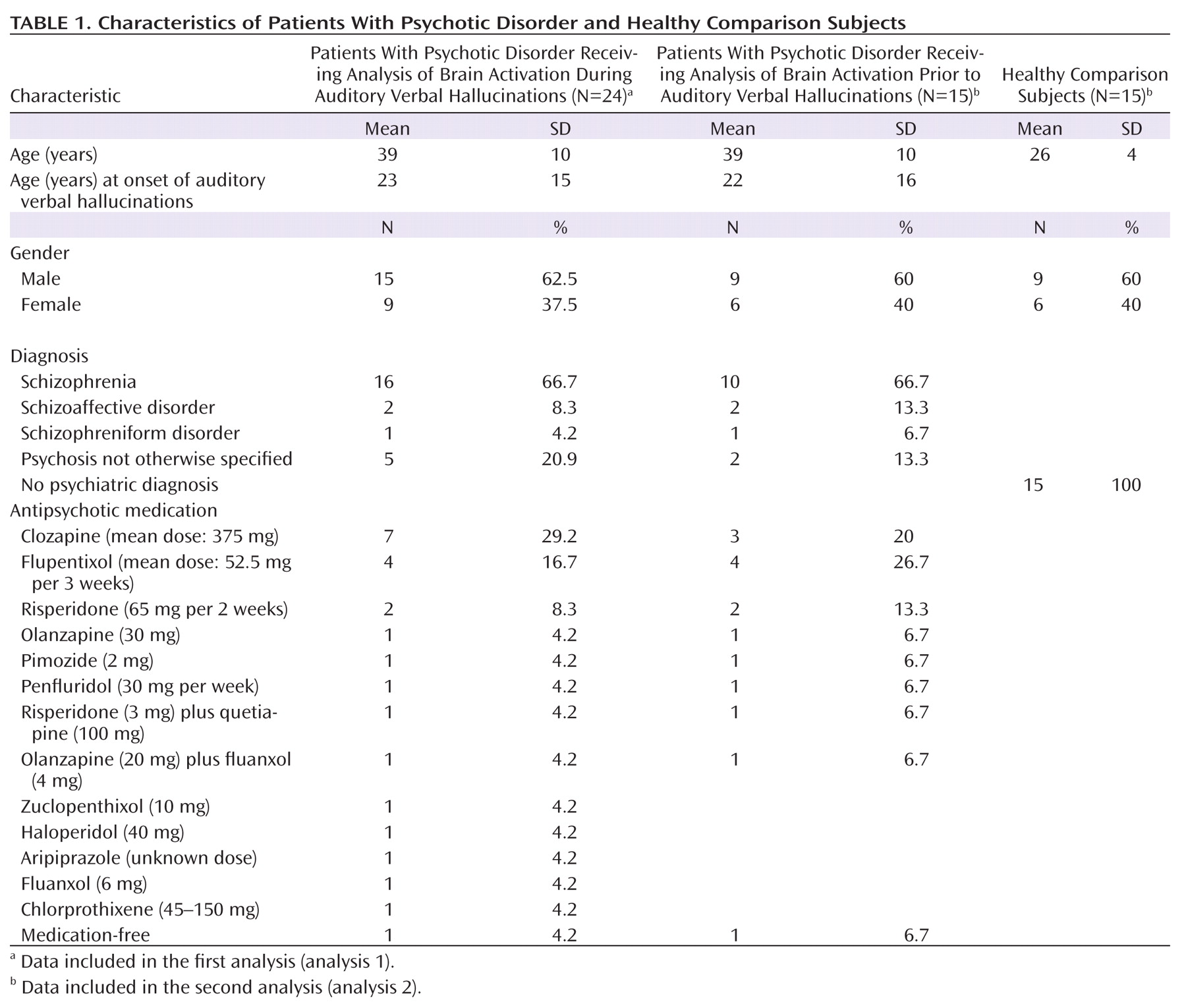
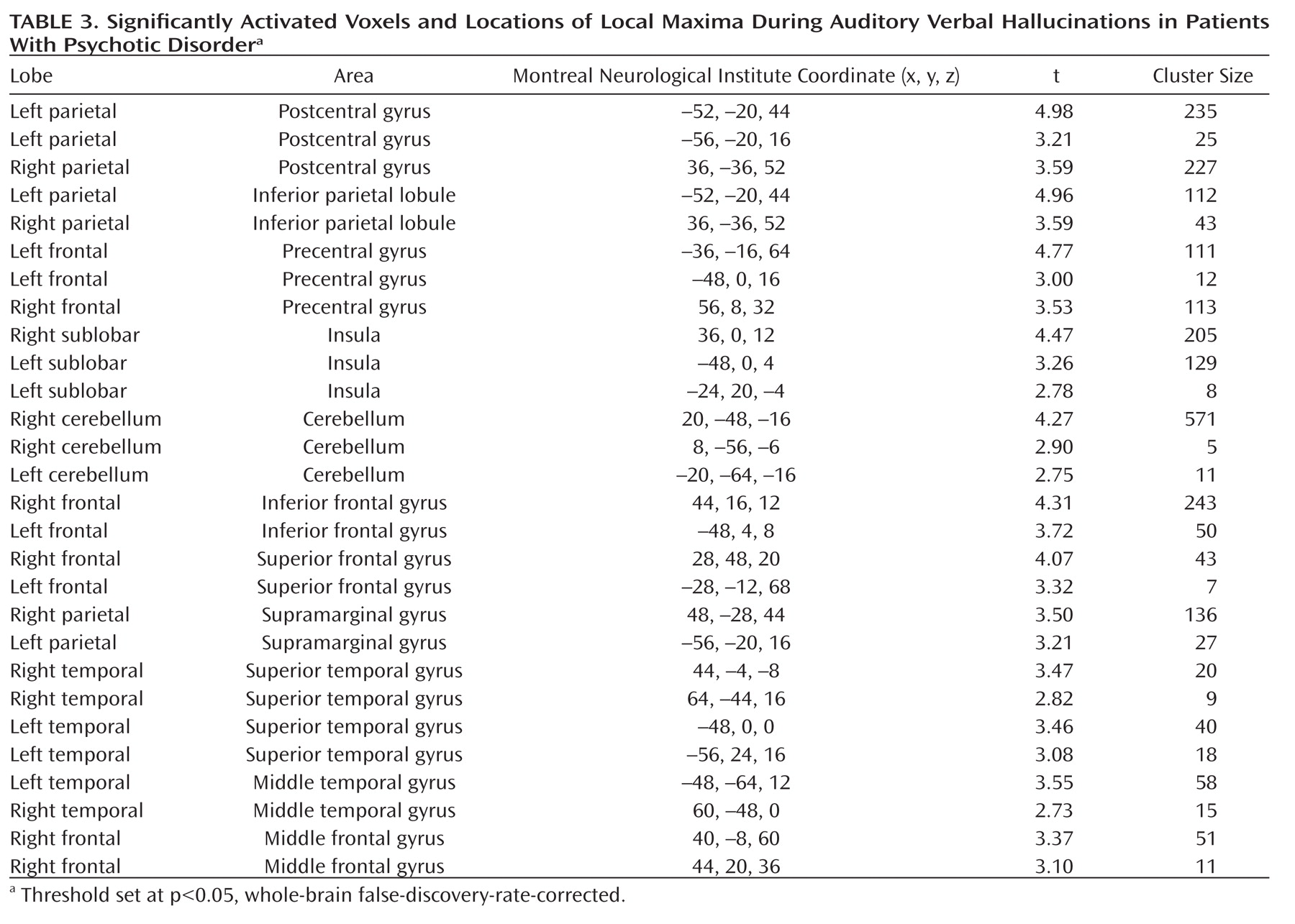
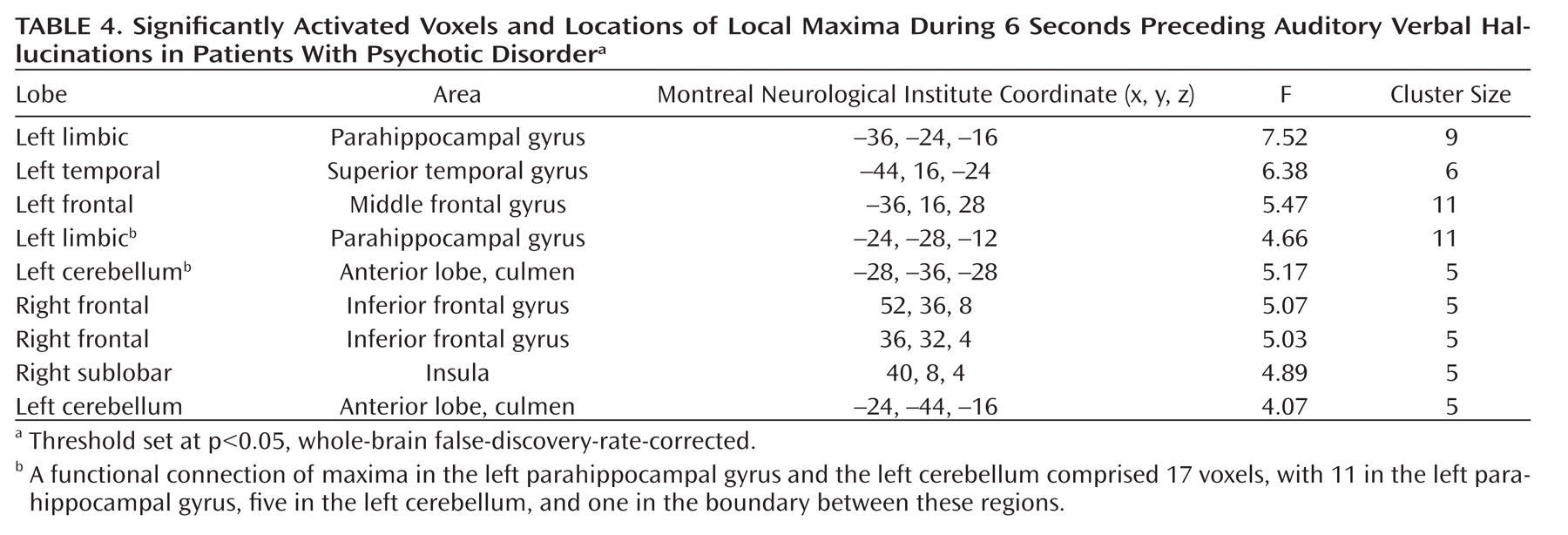
As seen in
Figure 1, the group analysis for hallucinations revealed activation of multiple confluent brain regions. These included language-related regions, such as the bilateral insula and inferior frontal gyrus (including Broca"s homologue) as well as the middle temporal, superior temporal, and supramarginal gyri. Other significantly activated regions consisted of the bilateral inferior parietal lobule, precentral gyrus, postcentral gyrus, cerebellum, and superior and middle frontal gyri.
For more clarity with respect to the different functional regions implicated in the group hallucination analysis, masks, created using the Automatic Anatomical Labeling Atlas (
19), were overlaid on the group results. Anatomic regions were chosen based on the locations of significantly activated local maxima in the groupwise hallucination analysis. Masks consisted of the bilateral inferior frontal, middle temporal, superior temporal, supramarginal, precentral, postcentral, middle frontal, and superior frontal gyri as well as the inferior parietal lobule, insula, and cerebellum. The amount of significantly activated voxels and the coordinates of the local maximum and its t values are reported for every masked region in
Table 3.
Analysis 2: Brain activation preceding auditory verbal hallucinations and random balloon squeezes
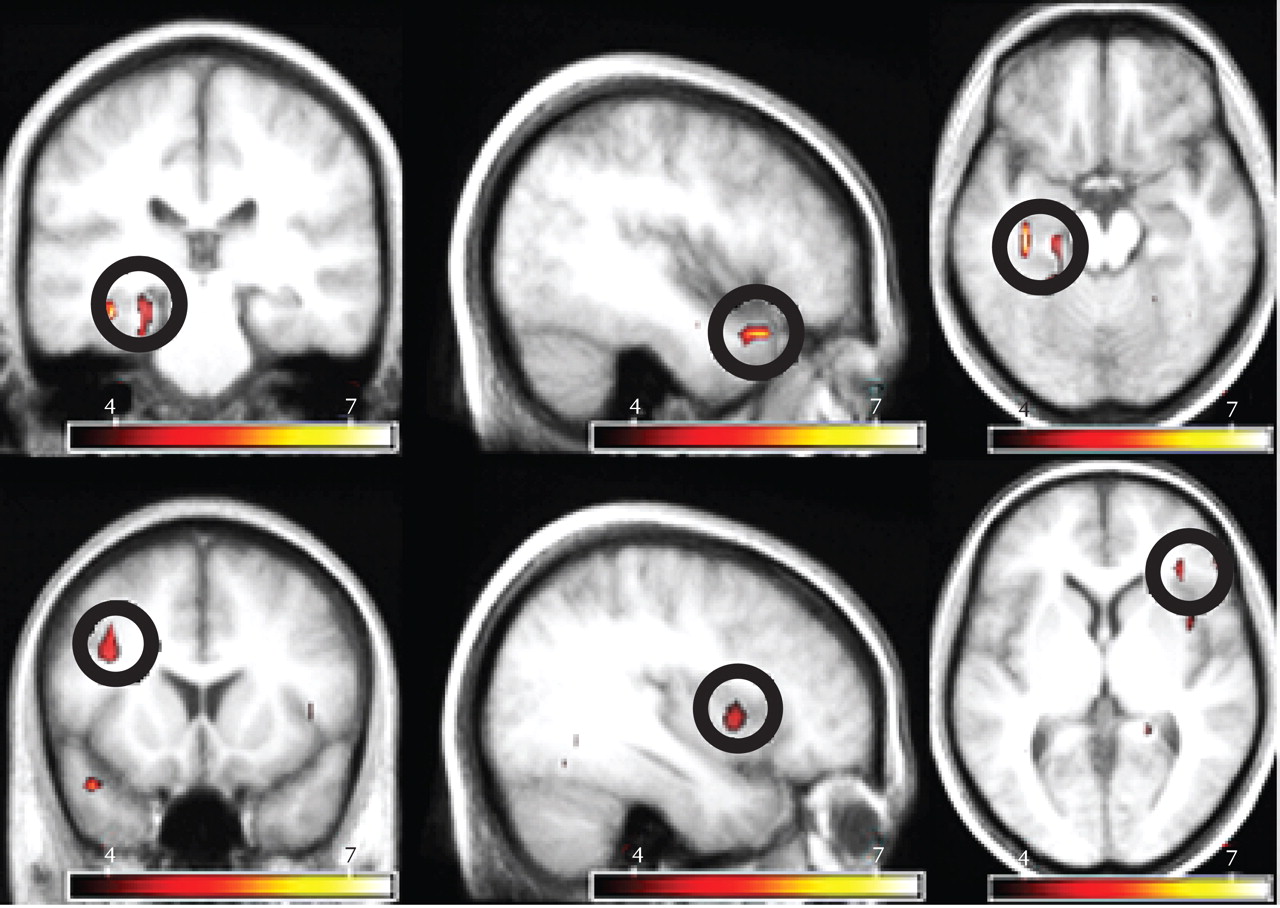
The most prominent signal change preceding hallucinations was observed in the left parahippocampal gyrus. In addition, significant signal changes were found in the left superior temporal, right inferior frontal, and left middle frontal gyri as well as the right insula and left cerebellum. The largest significantly activated cluster consisted of the following two interconnected local maxima: one located in the left parahippocampal gyrus and one in the left cerebellum. Since these areas are anatomically unconnected, a functional connection of these maxima probably resulted from spatial smoothing. Inspection of the cluster coordinates revealed that 11 voxels were located in the left parahippocampus, five were located in the left cerebellum, and one voxel was placed on the boundary between these regions.
Table 4 shows the coordinates of all significant local maxima in the group analysis. SPM2 F statistics are shown in
Figure 2.
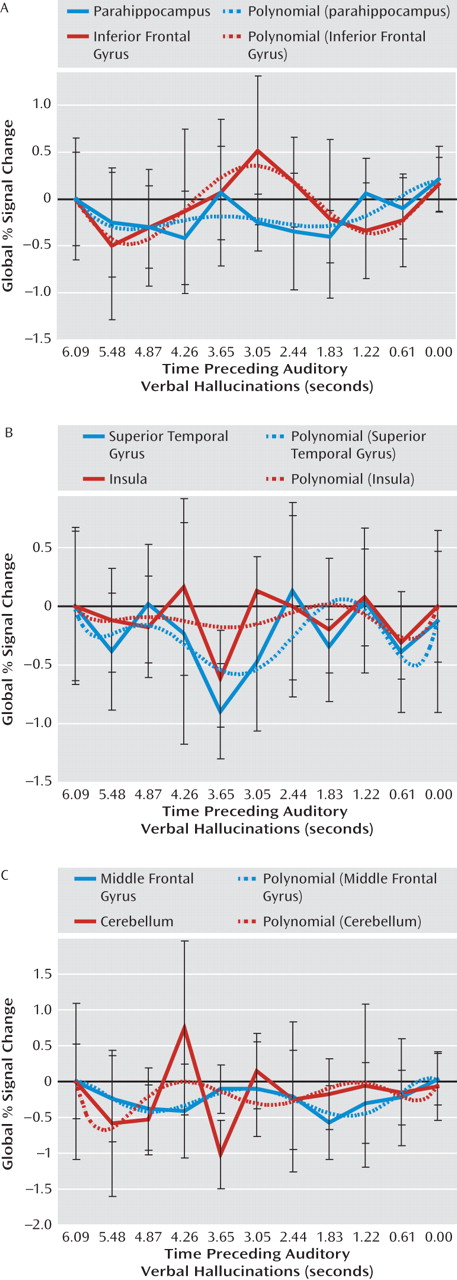
Figure 3 shows poststimulus time histogram plots from 6 to 0 seconds prior to hallucinations, averaged over all subjects, and a fitted sixth-order polynomial of all regions showing significant signal changes in the group analysis. From these poststimulus time histogram plots it can be concluded that the reported regions were significantly deactivated.
No significant effect of motion before the onset of hallucinations was revealed by the realignment parameters (for the three translation parameters as well as for the three rotation parameters), as identified by two separate repeated-measures MANOVAs, with the 10 scans preceding hallucinations as repeated measures.
Three independent repeated-measures ANOVAs, with gray and white matter and out-of-brain signal changes as dependent variables and the 10 scans preceding hallucinations as repeated measures, revealed no significant signal changes, indicating that motion artifacts cannot explain the effects detected with the finite-impulse-response analysis.
Brain Activation Preceding Balloon Squeezes
No significant signal changes were observed prior to the random balloon squeezes.
Discussion
This study investigated brain activation during auditory verbal hallucinations in 24 patients with psychosis. Brain activation during these hallucinations was primarily present in bilateral language areas. Brain activation preceding hallucinations could be investigated in 15 subjects. Groupwise analysis of signal changes up to 6 seconds preceding hallucinations showed pronounced deactivation in the left parahippocampal gyrus. Additional deactivation was observed in the left superior temporal, left middle frontal, and right inferior frontal gyri as well as the right insula and left cerebellum. These findings could not be attributed to motor activation, since groupwise analysis of activity in the 6 seconds prior to random balloon squeezes revealed no significant signal changes. Bilateral activation of language regions during hallucinations is consistent with a previous study conducted by our research group (
9).
Most prominent deactivation preceding hallucinations was observed in the parahippocampal gyrus. Because the parahippocampus is D
2 innervated (
20), this finding might represent an important link between dopaminergic overactivity and inadequate activation of bilateral language-related areas. The parahippocampus has been hypothesized to play a central role in memory recollection, since it receives perceptual information from association cortices, such as the language areas, and forwards this information to the hippocampus in order to be 'recognized.' This perceptual information is then passed back to the parahippocampal gyrus from where it is redistributed to the association cortices involved in the original perception (
21–
24). Indeed, activation of association cortices originally involved in the encoded fragment has consistently been shown during memory retrieval (
25–
27).
In the case of auditory verbal hallucinations, increased dopaminergic stimulation may enhance the redistribution function of the parahippocampal gyrus, leading to erroneous activation of an association cortex and hence to incorrect recognition. Disinhibition of the parahippocampal gyrus, demonstrated as deactivation preceding hallucinations, then triggers the bilateral language-related areas originally involved in the perception of speech fragments, as shown in the first analysis of the preset study. According to this hypothesis, hallucinations result from the spontaneous re-experience of memories, as already hypothesized in the nineteenth century (
28). Support for this hypothesis was provided in a study in which patients with hallucinations showed difficulties identifying the source of memories (
29). Furthermore, increases in hallucinations have been associated with an increasing inability to inhibit irrelevant memories (
30).
Deactivation instead of activation of the parahippocampus may seem at odds with its postulated role in memory recollection. However, deactivations have been reported to be realistic phenomena, probably caused by short decreases in neuronal activity (
31,
32). Previous studies on memory recollection have also reported deactivation of the parahippocampus (
33–
37).
Apart from parahippocampal deactivation, we observed significant deactivation preceding hallucinations in the left superior temporal and right inferior frontal gyri as well as right insula. These areas correspond with regions significantly activated during hallucinations and may result from the information redistributed to them by the parahippocampus, preparing them for activation in the course of hallucinations.
Our results are partially consistent with those of previous studies. Hoffman and colleagues (
38) reported deactivation of the parahippocampal and anterior cingulate gyri preceding hallucinations in six patients as well as activation of the left anterior insula and right middle temporal gyrus (
38). In addition, Lennox and colleagues (
39) found activity in the right middle temporal gyrus in a single subject, while Shergill and colleagues (
40) found activity in the left inferior frontal and right middle temporal gyri preceding hallucinations in two subjects. Deactivations were not discussed in these studies.
Limitations and Suggestions for Future Research
A limitation of this study is that most patients were treated with antipsychotic medication. Since the effect of antipsychotic medication is probably mediated by antagonism at the D
2 receptors (
2) and the parahippocampus is D
2 innervated (
20), parahippocampal function is expected to be influenced by D
2 blockade. The patients included in this study suffered from medication-resistant hallucinations. Therefore, deactivation of the parahippocampal gyrus is expected to be present in medication-free patients with psychosis prior to hallucinations, yet negative signal changes may be more pronounced in these patients. To further explore this effect, future studies should focus on brain activation preceding hallucinations in medication-free patients with psychosis.
Furthermore, a limitation of the finite impulse response can be that observed effects not necessarily reflect hemodynamic changes but instead any BOLD signal deviation from baseline. Nonetheless, in our study, analyses of signal changes in gray and white matter and out-of-brain voxels showed that movement artifacts could not have induced the observed deactivations because such artifacts should have been present in these regions also. Furthermore, the movement parameters describing head motion in the fMRI images did not differ significantly from zero before the hallucinations.
Since little a priori knowledge was available to indicate which neural and cognitive processes precede hallucinations, we selected a control condition that only controlled for motor preparation, enabling us to identify brain activation related to the actual hallucinations. A limitation of the control condition is that motor control was compared between subjects instead of within subjects. However, a within-subjects analysis was impractical as a result of the interference of hallucinations during random balloon squeezing among the patients.
In addition, a potential flaw is that significant deactivation preceding hallucinations may have resulted from subjects" delayed response to the hallucinations. In this case, peak deactivation would be expected to occur in the last seconds preceding hallucinations. However, in this study peak deactivation occurred between 4.87 and 2.44 seconds prior to hallucinations (Figure 3), which renders this explanation unlikely.
Finally, it can be argued that deactivation preceding hallucinations results from the 'poststimulus undershoot.' When applying BOLD fMRI, peak activation is followed by a short period of deactivation, which is the poststimulus undershoot. However, the poststimulus undershoot is typically present between 10 and 30 seconds after an event. Since the average time between successive hallucinations was 38 seconds in our study, this explanation also appears unlikely.
An important strength of this study is the large sample size and groupwise analyses. Furthermore, other explanations for the present findings were ruled out by including a comparison group and investigating the BOLD time course signals for motion artifacts. Finally, a particular strength is that an assumption-free finite-impulse-response model was used.
To test the model proposed in this study, future studies should focus on comparing hallucinations with word recall. An elegant design would be one in which patients recall their previous hallucinations