The hypothalamic-pituitary-adrenocortical (HPA) axis responds to threatening events with the release of glucocorticoids
(1) . These bind to receptors in specific areas of the brain such as the hippocampus, a region critical for certain types of memory. If the release of glucocorticoids is prolonged due to chronic stress, the hippocampus can sustain structural damage
(2), a proposed mechanism by which chronic stress adversely affects memory
(3 –
6) .
Measures of stress based on significant life events have been used to predict physiological and psychiatric changes in humans (e.g., major depression)
(7 –
10) as well as cognitive functioning. For example, Lupien and colleagues
(11) found that stressful experiences in a controlled laboratory setting resulted in a reduction in word recall in older, healthy individuals. Another study measuring naturally occurring life stresses in university students found that those reporting at least one life difficulty over the previous year recalled fewer words on a complex memory task than those reporting none
(12) . In contrast, Rosnick et al.
(13), in a large, population-based sample of older adults
(14), found that a self-report checklist of recent negative life events did not predict episodic memory performance, although one item, the recent death of a sibling, was associated with worse performance. A limitation of most of these studies is that few have examined the relationship between stress and memory change when both variables are measured repeatedly over time.
Other studies have investigated the way in which cortisol, a potential biomarker for stress, is associated with memory in older adults. Lupien and colleagues
(4) found that in healthy elderly subjects, an increase in plasma cortisol over time coupled with a high final level of cortisol predicted worse memory performance assessed after 4 years of follow-up. Csernansky et al.
(15) found that a higher level of plasma cortisol measured once during a follow-up period of up to 4 years predicted a faster rate of decline on a temporal lobe function factor that included tests of memory for participants with “preclinical” Alzheimer’s disease but not for cognitively intact participants. In another study, healthy community-dwelling individuals rated high on level of plasma cortisol performed worse on verbal memory tests in both the baseline and final sessions (18-month interval) than those rated low
(16) . Li et al.
(17) divided older individuals into quartiles based on evening salivary cortisol concentration measured at the beginning of the study and found a significant decline in delayed story recall over a 3-year period in the high cortisol group. Finally, in a community-based study, Seeman and colleagues
(18) found an association between increased urinary free cortisol (2.5-year interval) and memory decline for older women, but not for men. With the exception of this latter study, significant associations between cortisol and memory have been identified only when either cortisol or memory was measured at a single point in time.
Several findings link chronic stress and elevated cortisol to risk for dementia in elderly individuals. First, older adults at risk for Alzheimer’s disease by virtue of age are particularly vulnerable to hippocampal damage resulting from chronic stress
(6) . Second, recent animal studies have found stress and cortisol to be associated with neuropathological changes characteristic of Alzheimer’s disease including synapse loss
(19), increases in amyloid b-peptide (Ab)
(20,
21), tau accumulation
(20), and tau phosphorylation
(21) .
In the current study, we investigated whether prolonged exposure to stressful events and cortisol would predict changes in cognition (both global and memory) in normal elderly and those with mild cognitive impairment based on Petersen criteria
(22) . By investigating how measures of stressful events and cortisol levels assessed longitudinally are related to changes in memory, we sought to improve upon the current body of work in several ways. First, relatively few studies incorporate longitudinal data and measure the occurrence of specific events at multiple time points. Therefore, we measured stress at 6-month intervals and memory performance annually for up to 3 years in order to make valid inferences about changes in cognition during the period of observation. In addition, we used the Life Events and Difficulties Schedule
(23), an instrument with several advantages over simple event checklists including 1) strategies to increase reliability of participant reporting (e.g., written lists of events), 2) use of event context to determine threat severity and reduce subjectivity, 3) identification of event duration (short- versus long-term), and 4) documentation of multiple occurrences of events. Finally, multiple tests of episodic memory (story, word list, figure) allowed extensive examination of relationships between stress and memory.
Method
Participants
Volunteers (N=102) over the age of 65 and living independently were recruited from the UCSD Shiley-Marcos Alzheimer’s Disease Research Center and Memory Screening Clinic. Potential participants were excluded if found to have dementia, a significant medical condition (e.g., cancer; cardiac, pulmonary, or renal impairment; insulin-dependent diabetes; or chronic inflammatory disorders), or a significant psychiatric condition (e.g., moderate to severe depression), including posttraumatic stress disorder
(24) . Subjects using corticosteroid medications that could affect daily cortisol production were also excluded. Steroidal inhalants were permitted if stopped the day before and the day of sample collection. Topical corticosteroids were permitted. Other medications not known to disrupt HPA axis function were allowed if the participant had been on a stable dose for at least 6 months before the visit. These included nonsteroidal anti-inflammatory medications (used by 17% of participants), hormones (13%), narcotics (8%), anxiolytics (13%), and thyroid medications (23%).
According to a knowledgeable informant, each participant was functioning independently in daily activities (Pfeffer Outpatient Disability Scale
[25] ). Neuropsychological testing assessed memory, attention, executive functioning, visuospatial ability, and language. No participant met criteria for dementia as defined either by DSM-IV or NINCDS-ADRDA
(26) criteria for Alzheimer’s disease. Baseline cognitive functioning for 61 subjects was intact (normal cognition group); 41 participants showed deficits consistent with mild cognitive impairment based on Petersen criteria
(22) . The impairment in 24 of these subjects was classified as amnestic; 12 had nonamnestic single-domain impairment (executive functioning: N=9, visuospatial: N=3); and five were classified as having multiple-domain mild cognitive impairment.
Fifty-two participants were followed over a period of 1 to 3 years, with a mean follow-up interval of 2 years. Twenty-five were cognitively intact at baseline; 27 showed deficits consistent with mild cognitive impairment (amnestic: N=13; nonamnestic single-domain impairment: N=11; multiple-domain mild cognitive impairment: N=3).
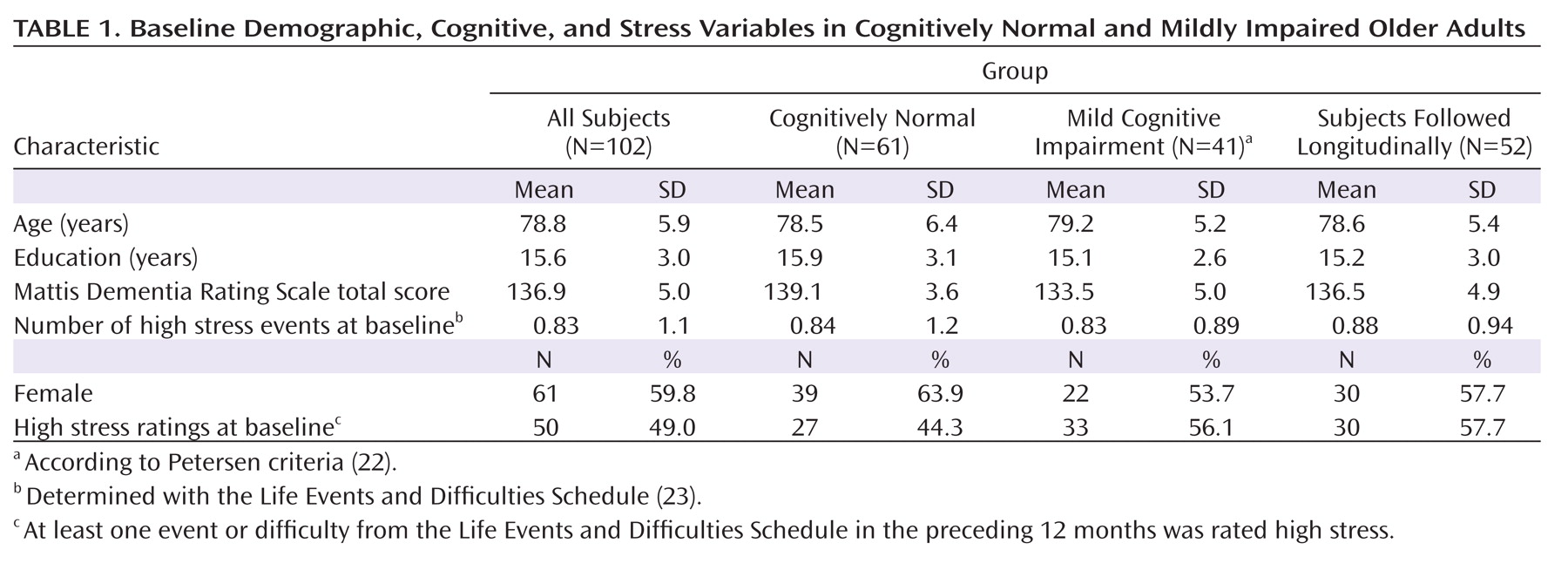
Sixty percent of the subjects were women; mean age was 78.8 years, and mean education was 15.6 years (
Table 1 ). Mean baseline score on the Mattis Dementia Rating Scale
(27) was 136.9 out of a possible 144 points. Subjects with mild cognitive impairment scored lower than cognitively normal subjects on the dementia rating scale (t=6.51, df=100, p<0.001), but there were no significant differences in age, education, gender, or number of high stress ratings at baseline. Subjects followed and not followed longitudinally did not differ significantly in age, education, gender, dementia rating scale score, or number of high stress ratings at baseline. When subjects receiving versus not receiving each type of medication (i.e., anti-inflammatory medications, hormones, narcotics, anxiolytics, thyroid medications) were compared using t tests, cortisol levels (i.e., intercepts and averages based on specific time of day) did not differ significantly. Using chi-square analyses, the number of subjects in the high and low cortisol groups did not differ significantly as a function of medication use.
Procedure
Measures of stress (i.e., Life Events and Difficulties Schedule ratings, cortisol level) were obtained at baseline and thereafter at 6-month intervals; neurological and neuropsychological evaluations were completed annually. A senior neurologist reviewed summary neuropsychological data and neurological findings at baseline to determine participant inclusion. Study personnel, including the reviewing neurologist, were blind to stress ratings and cortisol levels at the time of the evaluations.
After complete description of the study to each subject and informant, each signed a written informed consent approved by the UCSD Human Research Protections Program. The informant usually joined the participant for the Life Events and Difficulties Schedule interview, but in some cases, consented to provide information over the phone.
Assessment Instruments
The Life Events and Difficulties Schedule
(23) uses a semistructured interview method to identify chronic difficulties (>2-week duration) or discrete events serious enough to cause long-term threat. A list of events in 12 categories (e.g., finances, health
[28] ) helps the participant identify events (e.g., hospitalization) that occurred during the previous 12 months (baseline evaluation) and the previous 6 months (follow-up evaluations). Following methods described previously
(7), the interviewer probed for information about event context (e.g., duration) in order to construct a detailed description. Experiences covered include negative events (e.g., family death), transitions (e.g., first grandchild), and persisting difficulties (e.g., caregiving). The interviewer delivers summaries of reported experiences to a professional trained to determine degree of threat for each event/difficulty (high or low) using specific rules. An overall rating of “high stress” is assigned if there is at least one high stress event or difficulty within the period preceding that visit.
The Geriatric Depression Scale
(29) is a reliable and valid self-rating questionnaire for assessing depression in the elderly.
The State-Trait Anxiety Inventory
(30) measures trait anxiety defined as a tendency to perceive stressful situations as dangerous.
The Mattis Dementia Rating Scale
(27) assesses global cognitive functioning. Subtests include attention, initiation, visuospatial ability, conceptualization, and memory.
Episodic memory tests included 1) immediate and delayed recall from the visual reproduction (visual designs) and logical memory (stories) subtests of the Wechsler Memory Scale—Revised
(31), 2) list learning and retention from the California Verbal Learning Test
(32), and 3) verbal and visual material from the Dementia Rating Scale memory subscale.
Cortisol Measures
Sampling cortisol in saliva is a reliable, noninvasive method to assess circulating cortisol levels
(33) and HPA axis function. Participants used “Salivettes” (cotton swabs in plastic tubes) (Sarstedt, Rommeldorf, Germany) to produce samples within 1 day close to the time of the Life Events and Difficulties Schedule interview. Samples were refrigerated until delivered to the General Clinical Research Center Core Laboratory for analysis.
Cortisol EIA kits (Cat# 1–3002) were purchased from Salimetrics LLC (State College, Penn.). Samples, standards, controls, and Cortisol-HRP conjugate were added to a microplate coated with mAb to cortisol and incubated at room temperature for 1 hour; unbound components were washed and bound cortisol-HRP was measured using tetramethylbenzidine (TMB) substrate. The color was read on a Spectramax M-5 Plate reader. Unknown concentrations were determined using SMP 5.0 and a 5-parameter sigmoid minus curve fit. The intra- and inter-assay precisions were 0.01–2.5% and 3.0–8.0% respectively. The CV of duplicates varied from 0.01 to 2.5%.
Statistical Analyses
Cortisol measures from samples taken at five specified times (morning awakening, 30 minutes later, 2 p.m., 4 p.m., bedtime) on 1 day of each visit were log transformed to stabilize the variance. To explore the relationship between these diurnal measures of cortisol and cognitive outcomes, it was necessary to distill cortisol into a single measure for each visit. Options explored for this single measure included 1) the cortisol measure at a particular time of day (e.g., upon awakening), 2) the daily mean of all five cortisol measures per visit, and 3) change in cortisol during the day. Since the daily mean was the most informative, the per-visit measure of cortisol for each participant was defined as the mean of the five log-transformed cortisol values taken on that day. Individual rates of change in cortisol over time were examined as well but proved to be uninformative (approaching zero). Therefore, we used participant-specific intercepts as the summary measure of cortisol change. A random intercept linear model was fit to the longitudinal data for each participant with per-visit cortisol as a function of centered time. The resultant participant-specific intercepts are estimates of individual mean cortisol adjusted for the variable length of individual records. An additional binary indicator of high and low cortisol level was computed based on the median split of the intercept.
The same type of modeling was used to obtain per-participant life stress rating intercepts per the Life Events and Difficulties Schedule. These were used in analyses as adjusted estimates of an individual’s mean overall event-based stress level. To obtain per-participant cognitive rates of change, mixed effects linear models were fit with per-visit cognitive score as a function of time, with random (participant-specific) intercept and slope added to the model. These per-patient slopes were informative and therefore used in analyses as estimates of rates of change for cognitive measures.
Multiple linear regression analyses were performed with individual rates of cognitive change as the outcome. The main predictors were cortisol level intercept (expressed as a high/low binary indicator per median split), life stress rating intercept (per the Life Events and Difficulties schedule, expressed as a high/low binary indicator per median split), baseline diagnosis (cognitively normal/mild cognitive impairment), and two interaction terms (baseline diagnosis-by-cortisol intercept; baseline diagnosis-by-life stress rating intercept), with gender as a controlling covariate. Age, education, and measures of depression and anxiety were tested for inclusion as covariates but were not found to be significant. Throughout the analyses, a negative slope signifies decline in the cognitive score, with magnitude of decline indicated by the size of the slope. For example, a slope of –0.61 for the Dementia Rating Scale memory subscale indicates a decline of 0.61 points during the observation period.
Results
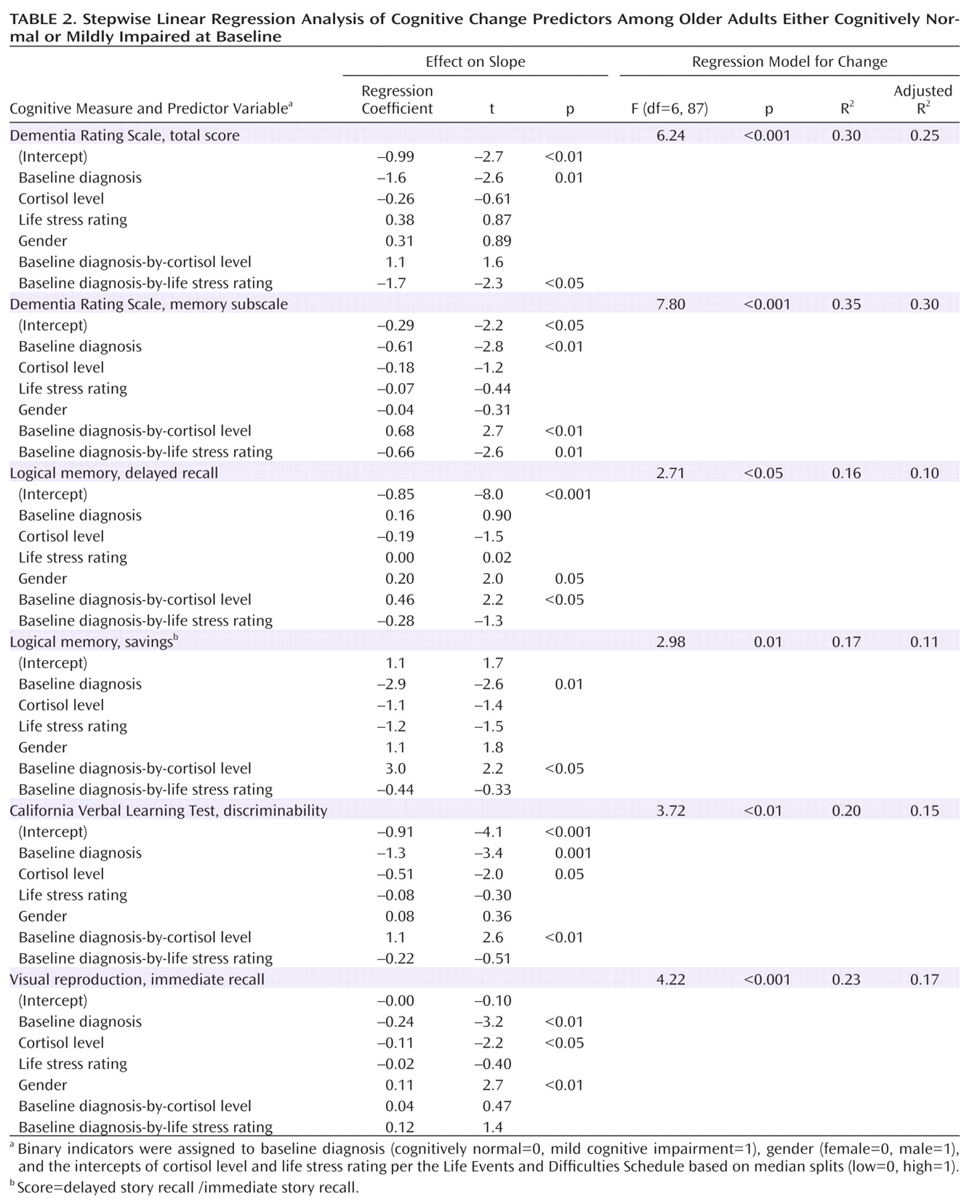
There were no significant correlations between cortisol level and life stress rating intercepts for the cohort as a whole or separately for the cognitively normal and mild cognitive impairment groups. Therefore, these stress measures were treated as independent factors in subsequent analyses. Cortisol level, life stress rating, baseline diagnosis, and gender were combined in linear regression analyses to predict change (i.e., slopes) in each cognitive measure. Main effects and interactions of baseline diagnosis with both life stress and cortisol measures were assessed (
Table 2 ).
The regression model for change in Dementia Rating Scale total score was significant and included a main effect for baseline diagnosis and an effect for the interaction of diagnosis and life stress rating. A diagnosis of mild cognitive impairment predicted faster decline on the Dementia Rating Scale over follow-up, and having both a mild cognitive impairment diagnosis and a high intercept for the Life Events and Difficulties Schedule rating (greater stress) predicted an additional increase in rate of decline.
The regression model for change in Dementia Rating Scale memory subscale score was also significant and yielded a main effect for baseline diagnosis and interaction effects for diagnosis-by-life stress rating and diagnosis-by-cortisol level. A diagnosis of mild cognitive impairment predicted faster decline on the Dementia Rating Scale memory subscale. A mild cognitive impairment diagnosis and high stress rating per the Life Events and Difficulties Schedule predicted an additional increase in rate of decline. In contrast, a mild cognitive impairment diagnosis and high cortisol level predicted a decrease in rate of decline.
The regression model for change in the logical memory delayed recall score revealed a main effect for gender (male associated with slower decline) and a baseline diagnosis-by-cortisol level interaction effect. Having a mild cognitive impairment diagnosis and high cortisol intercept predicted a decrease in rate of decline. The model for change in the logical memory savings score was also significant, with a diagnosis of mild cognitive impairment associated with an increase in rate of decline, but a diagnosis of mild cognitive impairment coupled with high cortisol being associated with a decrease in rate of decline.
The regression model for change in the California Verbal Learning Test recognition discriminability index revealed main effects for baseline diagnosis (mild cognitive impairment associated with faster decline) and cortisol level (high intercept associated with faster decline). There was also a significant diagnosis-by-cortisol interaction effect, with the combination of a mild cognitive impairment diagnosis and high cortisol associated with a decrease in rate of decline.
Finally, the regression model for change in the visual reproduction immediate recall score showed main effects of diagnosis (mild cognitive impairment associated with faster decline), cortisol level (high intercept associated with faster decline), and gender (male associated with slower decline), but no interaction effects. In addition, there were no significant main or interaction effects in regression analyses predicting slopes for California Verbal Learning Test long delay free recall and savings scores.
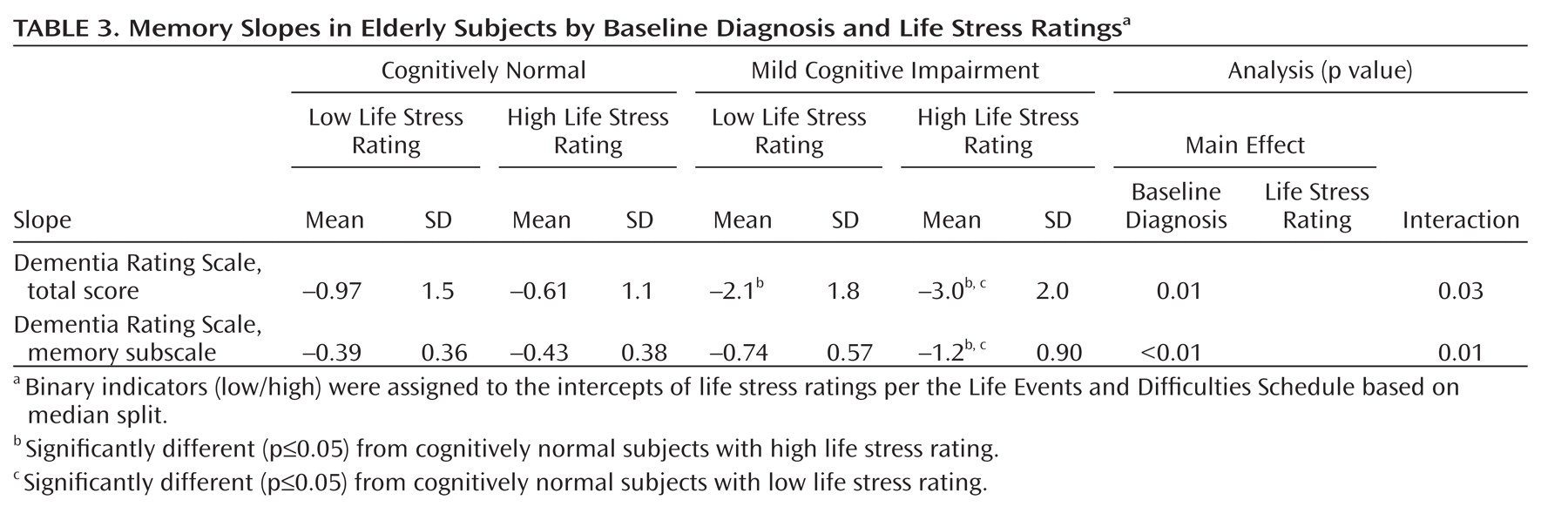
Mean slopes for the Dementia Rating Scale total and memory measures are presented as a function of baseline diagnosis and life stress rating (
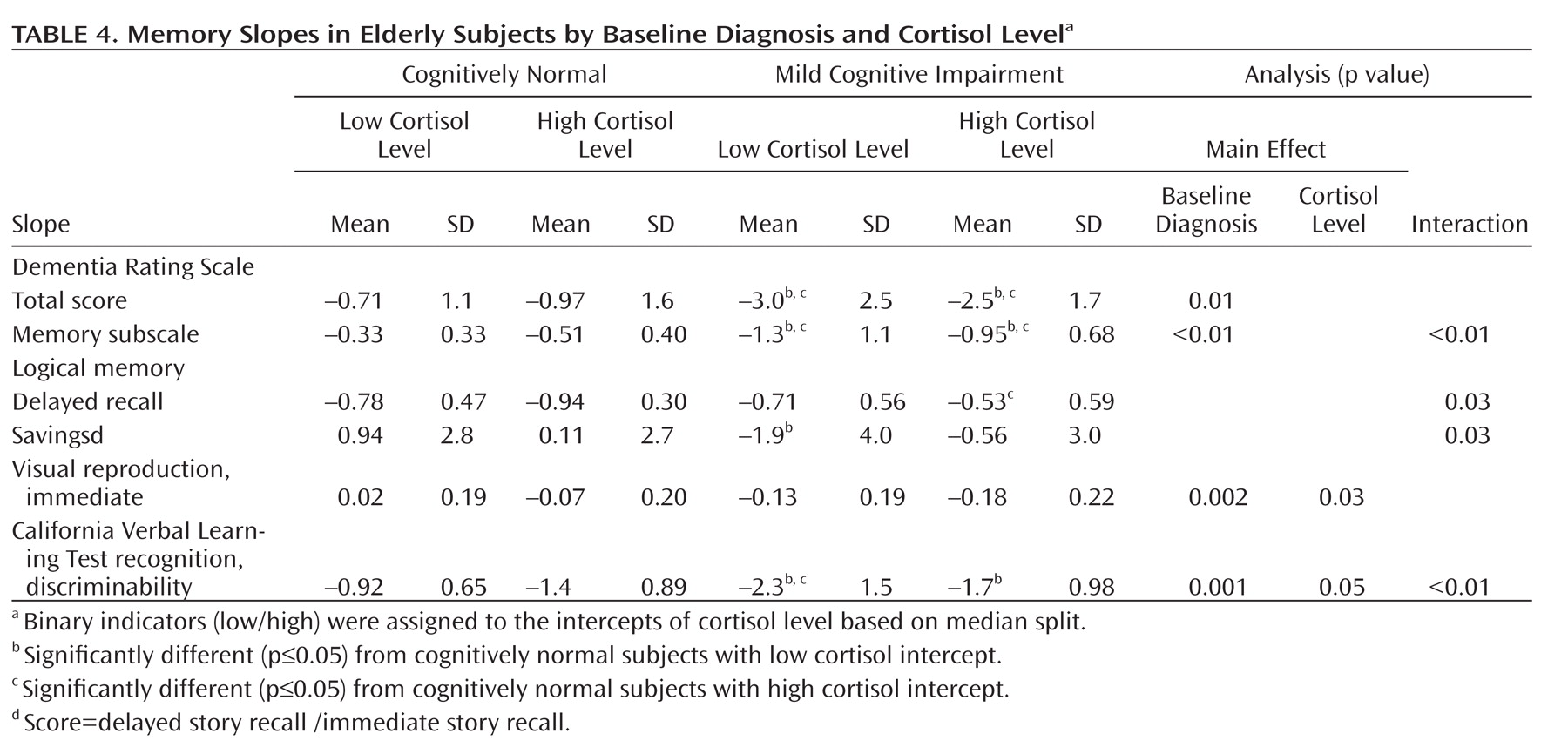
Table 3 ) and baseline diagnosis and cortisol level (
Table 4, only significant main or interaction effects based on univariate ANOVA are shown). Finally, similar to results from Seeman et al.
(18), we found a significant association between higher average cortisol levels and a faster rate of decline (California Verbal Learning Test savings score slope) for women (Pearson r=–0.32, p<0.02), but not for men.
Discussion
Study results indicate that the presence of stressful life events over a period of up to 3 years is associated with accelerated cognitive decline in older adults with compromised cognition (i.e., mild cognitive impairment). Subjects with mild cognitive impairment declined more rapidly than cognitively normal subjects on almost all cognitive measures, but this decline was exacerbated for Dementia Rating Scale total and memory subscale scores in mild cognitive impairment subjects with high stress ratings per the Life Events and Difficulties Schedule. Life stress ratings, however, were not associated with accelerated cognitive decline in cognitively normal subjects. Methodological differences (e.g., acute versus chronic stress; event checklist versus extended interview) may explain inconsistencies between our results targeting cognitively normal subjects and results of previous studies finding a negative association between stress and memory
(11 –
13) in “cognitively intact” or “nondemented” subjects. Also, previous studies may not have carefully excluded subjects with mild cognitive impairment. Given our results, it may be that stress (based on life events) does not affect memory in subjects with clearly intact cognition, but does if brain function is already compromised. Because subjects with mild cognitive impairment may be in a preclinical state of Alzheimer’s disease, we would expect them to be more vulnerable to the effects of stress on cognition.
The association of increased cortisol level with a
decreased rate of cognitive decline in subjects with mild cognitive impairment over the observation period was an unexpected finding. This effect was consistent for all four memory measures that showed a significant baseline diagnosis-by-cortisol level interaction effect, suggesting that cognitively normal subjects and subjects with mild cognitive impairment differ in the ways that cortisol affects the brain substrates of memory (e.g., hippocampus). A number of possible explanations for this effect in subjects with mild cognitive impairment should be considered. First, a beneficial effect of cortisol on vigilance and attention
(16,
34) may provide some compensation on memory tasks performed by someone with compromised memory. Second, some subjects with mild cognitive impairment aware of their cognitive decline may exert “stressful” effort in order to maintain cognitive functioning and, as a result, increase their level of cortisol. Third, there is little consensus in the literature regarding an absolute level of cortisol sufficient to cause hippocampal change, and the cortisol values for the cognitively normal subjects and subjects with mild cognitive impairment in the higher cortisol groups in our study (6.9 and 6.8 nmol/liter respectively) may not have been high enough to cause memory loss. Nevertheless, high cortisol levels would not be expected to have the observed beneficial effect in subjects with mild cognitive impairment. It is possible that subjects with mild cognitive impairment had memory loss severe enough at baseline to cause a floor effect resulting in a flatter slope with repeated testing. This, however, would not fully explain the diagnosis-by-cortisol interaction, since a similar floor effect would be expected in both low and high cortisol groups. Perhaps the most likely possibility is that existing hippocampal damage in subjects with mild cognitive impairment changes the impact of cortisol on hippocampal function and memory. A previous study
(35) found that prolonged corticosterone treatment in rats prescreened for cognitive impairment (water maze) had a significant negative effect on performance on subsequent learning and memory in the “nonimpaired” group but no significant effect in the “impaired” group. Histopathological analysis showed a much greater difference in cell damage in the CA1 region of the hippocampus before and after corticosterone administration in the “nonimpaired” rats (450% increase) than in the “impaired” rats. These results suggest that the magnitude of cortisol-induced change in the hippocampus may be a critical factor underlying reduced vulnerability that we observed in the “impaired” group with mild cognitive impairment relative to the “nonimpaired” cognitively normal group.
Interestingly, we did not find the expected positive relationship between life event ratings per the Life Events and Difficulties Schedule and salivary cortisol level. According to Hellhammer and colleagues
(36), this is not surprising given the methodological imprecision associated with simple checklist measures of stress and the vast array of factors that link stress to HPA axis activation. Similarly, Michaud et al.
(37) suggest that interpretation of stress/cortisol associations may depend on specific types of stressors and individual coping mechanisms. Given these complexities, targeting other measures of HPA axis functioning may improve our ability to predict the effects of prolonged stress. For example, corticotropin-releasing hormone (CRH), like cortisol, regulates neuroendocrine responses to stress and is expressed, along with its receptors, in a number of brain regions including the hippocampus. CRH, however, may be a more general mediator or integrator of adaptation to stress than cortisol. Several animal studies
(38,
39) have demonstrated a significant adverse impact of CRH on hippocampal structures in response to stress, and some
(39,
40) have speculated that glucocorticoids are involved to a lesser degree than had been assumed.
Several limitations of our study should be noted. First, the high mean education level of the participants somewhat limits the generalizability of the results. Second, we addressed a number of factors that could influence how stress affects cognitive abilities (e.g., age, education, depression and anxiety, gender, diagnosis, event duration), but not others (e.g., coping traits, genetic status, early life stress). Third, sample sizes for the two groups provided adequate power to demonstrate that the presence of stressful life events is associated with accelerated cognitive decline in individuals with mild cognitive impairment. It is possible, however, that additional effects (e.g., relationship between measures of cortisol and stressful events) would have been found with larger sample sizes. Replication of these results in larger, independent subject samples is necessary to confirm the current positive and negative findings. Finally, follow-up extending beyond 2 to 3 years may be necessary to identify a critical point after which neural recovery in response to waning stress is no longer possible. Despite limitations, the present results demonstrate associations among chronic event-based stress, salivary cortisol concentrations, and memory decline in older adults. Better models to understand how HPA axis responses to stress influence specific brain structures and pathways associated with memory in older adults could improve our ability to intervene before additional neuropathological changes occur.