The definition of neurophysiological and molecular markers of schizophrenia is of special importance. According to Turetsky et al. (
1), patients with schizophrenia exhibit deficits in the inhibition of responses to redundant stimuli and in the enhancement of responses to novel stimuli. Impaired inhibition can be characterized by prepulse inhibition of the startle reflex, P50 auditory evoked potential suppression, and antisaccade eye movements, whereas mismatch negativity and P300 event-related potentials are the markers of novelty detection (
1). The deficit of P50 suppression was the first objective phenotype successfully linked to a candidate gene locus, the β7-nicotinic cholinergic receptor subunit gene on chromosome 15q14 (
2,
3). The essence of sensory gating and inhibition is that the nervous system adapts to repeated stimulation in order to "gate" out irrelevant information and to avoid sensory overload (
4). During the auditory dual-click paradigm, two consecutive clicks are presented, and a positive-polarity brain response appearing 50 msec poststimulus, the P50 wave, is recorded. Decreased P50 amplitude to the second click relative to the first characterizes the strength of sensory gating and recurrent inhibition (
1–
4). Three large meta-analyses demonstrated that deficient P50 suppression is a robust marker of schizophrenia, although clinical and demographic variations of participants and methodological differences have led to heterogeneous results (
5–
7). Evidence also suggests that the P50 inhibition deficit is a possible endophenotype, since it was observed in nonpsychotic biological relatives of patients with schizophrenia and was linked to a candidate gene (
1–
3). However, de Wilde et al. (
8) failed to find P50 suppression deficits in young first-episode patients with schizophrenia and in clinically unaffected siblings. The P50 inhibition deficit is consistent with the current classification of schizophrenia and bipolar disorder, although bipolar patients with a history of psychosis also show diminished P50 inhibition (
9,
10).
Although current research is mainly focused on the relationship between neurophysiological endophenotypes and candidate genes, nongenomic molecular correlates of physiological abnormalities are also important. In this respect, intracellular signaling networks, which integrate information from the receptors of neurotransmitters and growth factors implicated in schizophrenia, are of special relevance. The phosphoinositide 3′-kinase (PI3K)/protein kinase B (AKT)/glycogen synthase kinase (GSK)-3β system is modulated by dopamine, glutamate, neuregulin 1, dysbindin, and Disrupted in Schizophrenia-1, and it represents a candidate pharmacological target for antipsychotics and mood stabilizers (
11–
16).
Neuregulin 1 and its receptor play an important role in neurodevelopment and synaptic plasticity via the activation of the PI3K-AKT pathway regulating cell migration and adhesion (
17,
18), which are potentially relevant mechanisms in the pathogenesis of schizophrenia (
13,
14). At the physiological level, neuregulin 1 modulates sensory gating. Liu et al. (
19) showed that neuregulin 1 increases β7-nicotinic acetylcholine receptors and enhances synaptic transmission in hippocampal inhibitory interneurons. Zhong et al. (
20) demonstrated that type III neuregulin 1 enhances ventral hippocampal-accumbens transmission by nicotine. Neuregulin 1 also affects fast gamma oscillations in the hippocampus (
21), which may be linked to P50 gating (
22; but see also Grunwald et al. [
23]). Therefore, it is plausible to hypothesize that neuregulin 1-related signaling and P50 gating are linked in schizophrenia. In a clinical setting, neuregulin 1-related signaling can be studied using peripheral markers. Sei et al. (
13) found that neuregulin 1-induced migration of B lymphoblasts of patients with schizophrenia is significantly decreased relative to comparison subjects. Impaired migration was related to smaller neuregulin 1-stimulated AKT phosphorylation and was associated with polymorphisms of the neuregulin 1 and catechol-
O-methyltransferase genes (
13). The robust reduction of neuregulin 1-induced AKT phosphorylation is a promising peripheral marker, and AKT-related signaling may be relevant to the pathophysiology of schizophrenia (
24–
26).
Based on these data and assumptions, the present study had the following specific aims and hypotheses. 1) We intended to replicate the P50 gating deficit in first-episode, young, never-medicated patients with schizophrenia. The hypothesis was that these patients would exhibit P50 suppression deficits. 2) We measured neuregulin 1-induced AKT phosphorylation in the peripheral B lymphoblasts of these patients. The hypothesis was that the patients would display decreased AKT neuregulin 1-induced phosphorylation relative to comparison subjects. 3) Finally, the relationship between P50 gating and neuregulin 1-induced AKT phosphorylation was investigated. The hypothesis was that less efficient neuregulin 1 signaling (lower AKT phosphorylation) would be associated with more impaired P50 gating.
Method
Participants
Thirty patients with first-episode schizophrenia and 30 healthy comparison volunteers participated in the study (21 men and nine women in both groups). The patients had a first episode of schizophrenia per DSM-IV criteria and had not received psychotropic medications. The healthy participants were university/hospital employees and their nonbiological relatives and acquaintances. All participants received the Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) (
27). The healthy participants had no axis I psychiatric disorders and had a negative family history for psychotic disorders. Of the 30 patients with schizophrenia, six were smokers and 24 were nonsmokers. The prevalence of smoking was the same in the comparison group. Detailed medical records were available for each patient in which the report of parents and caregivers was depicted. In addition, we interviewed one of the relatives of each patient. Exclusion criteria were neurological disorders, head injury, history of psychoactive substance dependence, and positive toxicological screening. The Positive and Negative Syndrome Scale (PANSS) (
28) was used for the assessment of clinical symptoms. The mean duration of untreated psychosis was 9.6 months (SD=4.3). The demographic and clinical characteristics of the subjects are depicted in
Table 1. The study was carried out in accordance with the Declaration of Helsinki and was approved by the local ethics board. All participants were able to give written informed consent, which was confirmed by two independent psychiatrists not included in the study.
Electrophysiological Recordings
The measurements were performed between 10:00 a.m. and 12:00 p.m. The participants were in a comfortable supine position and fixated to a target point to maintain alertness. The participants did not smoke cigarettes 24 hours before the experiment to avoid the enhancing effect of nicotine on P50 inhibition (
29). The active gold disk electrode was affixed to the vertex (Cz), and the reference electrode was affixed to the ears. The EEG was amplified 20,000 times with bandpass filters (–50%) between 1 and 300 Hz. The electrooculogram (EOG) was recorded with electrodes placed at the right superior orbit and lateral canthus. For each paired stimuli (two consecutive clicks), the duration of recordings was 1000 msec (intrapair interval=0.5 second; interstimulus interval=10 seconds). The duration of the square wave pulse was 0.04 msec amplified in the 20–12,000 Hz bandwidth. The stimuli were delivered through earphones (duration: 2.5 msec, intensity: 70 dB). If a startle response was present, the sound intensity was reduced by 2 dB. If the test wave amplitude was smaller than 1.0 μV, the sound intensity was increased by 2 dB. At least five average evoked potential recordings of 16 trials were collected for each participant. The digital filters were a high-pass filter (10 Hz) and a seven point, low-pass filter (300 Hz; A=0.95). The grand averaged analysis excluded recordings with no measurable conditioning P50 waves or with prominent EOG activity at the same latency as the P50. There were no differences in the number of trials for the patient and comparison groups. The P50 wave was identified and analyzed as described by Nagamoto et al. (
30). The most positive pair was selected between 40 and 75 msec after the conditioning stimulus. The test P50 wave was the most positive waveform, ±10 msec of the latency of the conditioning P50 component. A trained experimenter blind to the diagnosis reviewed all recordings. The amplitude was measured relative to the previous negativity. P50 suppression was calculated as test wave amplitude/conditioning wave amplitude. We also determined the difference between the conditioning and test wave amplitude (
31).
Neuregulin 1-Induced AKT Phosphorylation
The protocol of Sei et al. (
13) was used. B lymphocytes in the mononuclear cell preparation were transformed by infection with Epstein-Barr virus. The lymphoblasts were then stimulated with neuregulin 1a for 30 minutes (296-HR, 65 amino-acid residue recombinant protein from the epidermal growth factor domain of neuregulin 1a [177–241]; R&D system Inc., Minneapolis; 200 ng/ml). The protein isolated from the cells was analyzed by Western blotting. To quantify the level of phosphorylation, the immunoblots were stained with antibodies specific to phosphorylated AKT1 at Ser473 (60 kDa) and were reprobed with total AKT (both antibodies were purchased from Upstate, Charlottesville, Va.; 1 mg/ml). For the immunoblot analysis, cells were disrupted in Bioforce homogenizers (buffer: 250 mM Tris-HCl, 100 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% Deoxycholic acid, 1 mM p-nitrophenylguanidinobenzoate, and protease inhibitor cocktail I and II [Sigma]). The homogenized material was incubated for 20 minutes at 41°C, and then was centrifuged (14000g for 15 minutes) to obtain supernatants for further analysis. Following the collection of supernatants, the protein samples were separated (SDS-PAGE on Tris-glycine gel [Invitrogen, Carlsbad, Calif.]), transferred to polyvinylidene difluoride membrane (Millipore Corp, Bedford, Mass.), and then probed with the primary antibodies. The primary antibodies were detected using horseradish peroxidase-conjugated antirabbit IgG antibodies (Promega, Madison, Wisc.), which was followed by enhanced chemiluminescence detection (Amersham Biosciences, Buckinghamshire, U.K.). The protein bands within the linear range of the standard curve were imaged. The relative optical density was measured using the National Institute of Health Image software. The dependent measure was the ratio of phosphorylated AKT to total AKT at baseline (without neuregulin 1 stimulation) and after neuregulin 1 stimulation. The phosphorylated form/total ratio was calculated after a quantitative densitometric analysis of each band within a linear range (
13).
Data Analysis
The STATISTICA Release 8 (StatSoft, Inc., Tulsa) software was used for data analysis. First, the normality of data distribution and the homogeneity of variance were examined by Kolmogorov-Smirnov and Levene's tests, respectively. If the normal distribution or the homogeneity of variance was violated, nonparametric Mann-Whitney U tests were used. If not, repeated measures analysis of variance (ANOVA), followed by Scheffé's post hoc tests, or two-tailed t tests were used. In the correlation analysis, Spearman's correlation coefficients were calculated. The level of significance was set at a<0.05.
Results
AKT Phosphorylation
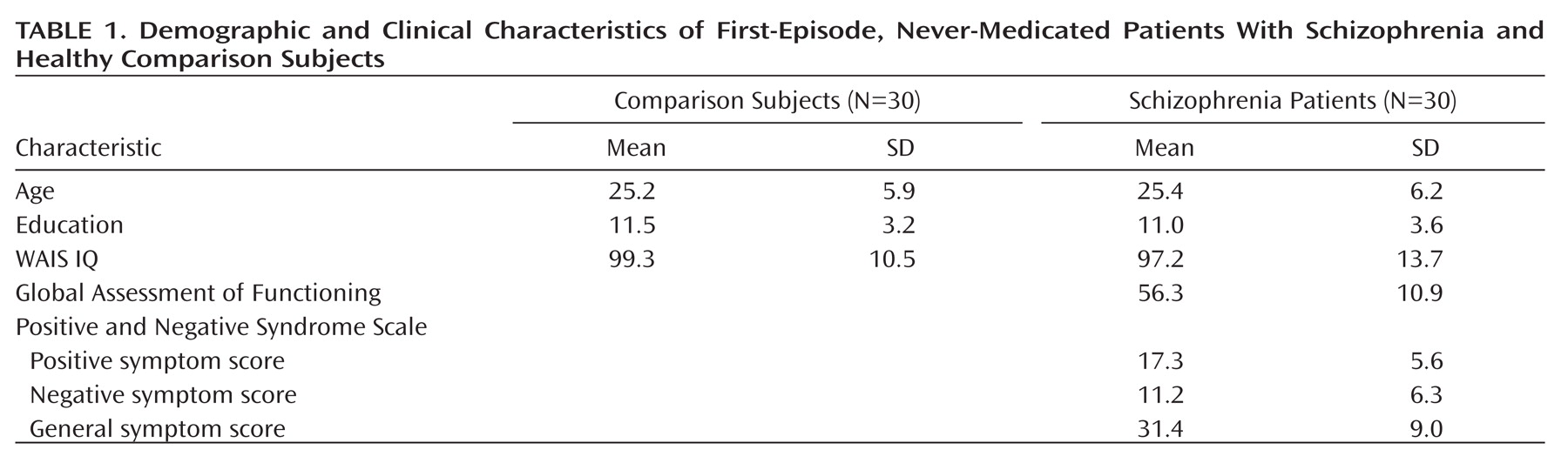
The phosphorylated AKT to total AKT ratios are shown in
Figure 1. The ANOVA conducted on the ratios revealed significant main effects of group (F=31.70, df=1,58, p<0.0001) and neuregulin 1 stimulation (F=11.06, df=1, 58, p<0.01). The interaction between group and neuregulin 1 stimulation was significant (F=29.89, df=1,58, p<0.0001). Scheffé's tests indicated a significantly increased phosphorylated AKT to total AKT ratio in the comparison group after neuregulin 1 stimulation (p<0.0001), which was not observed in the patient group (p=0.52). In the baseline condition, patients with schizophrenia did not differ from comparison subjects (p=0.82), whereas in the neuregulin 1-stimulated condition, patients exhibited a significantly lower phosphorylated AKT to total AKT ratio relative to comparison subjects (p<0.0001). There were no significant differences between smokers and nonsmokers (p>0.10).
P50 Suppression
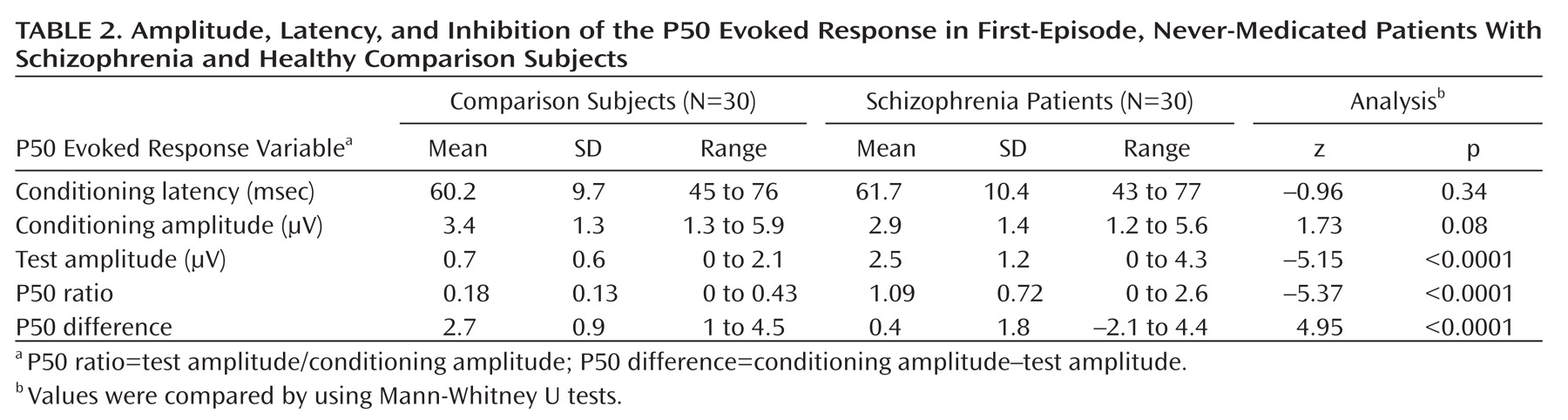
Table 2 shows the mean amplitude, latency, suppression index, and absolute difference of the P50 evoked potential. As revealed by Mann-Whitney U tests, patients with schizophrenia displayed a significantly decreased P50 suppression relative to the comparison subjects. There were no significant differences in latency and conditioning wave amplitude. There were no significant differences between smokers and nonsmokers (p>0.10).
Correlations Between P50 Suppression and AKT Phosphorylation
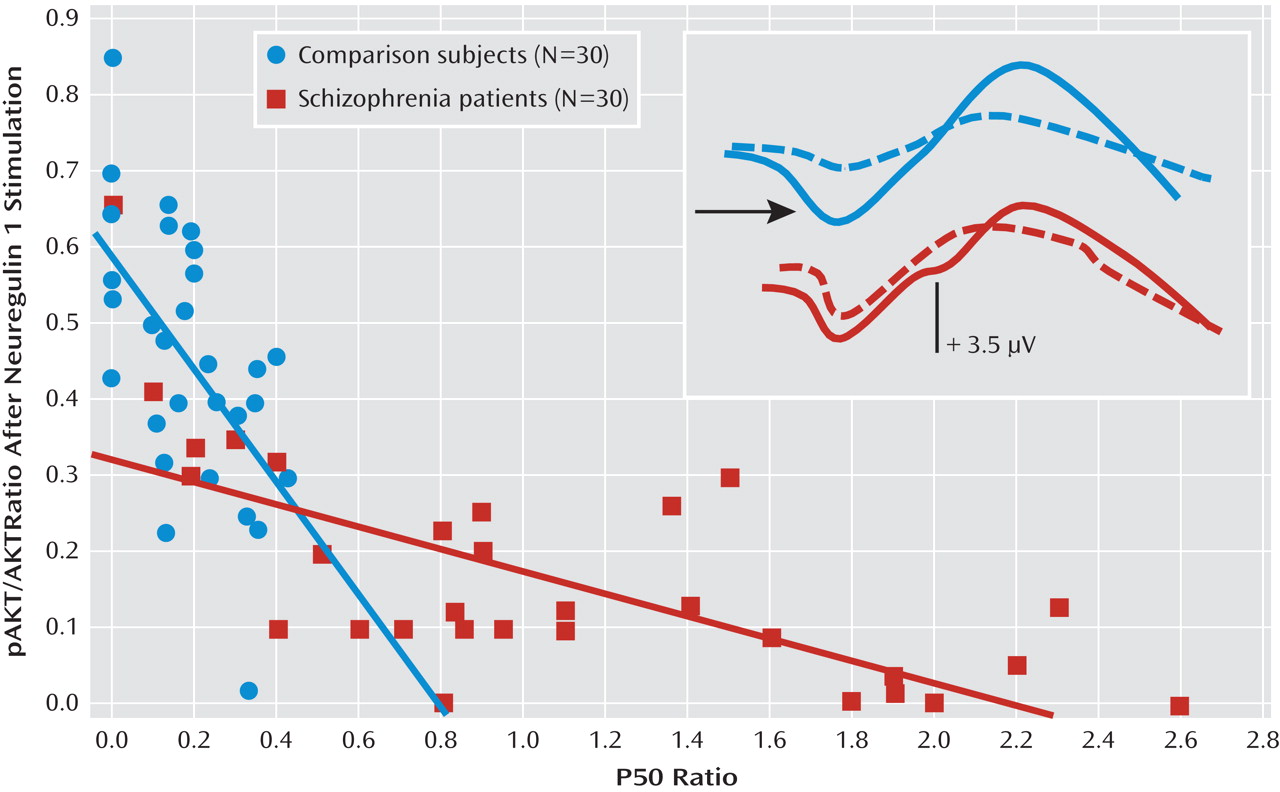
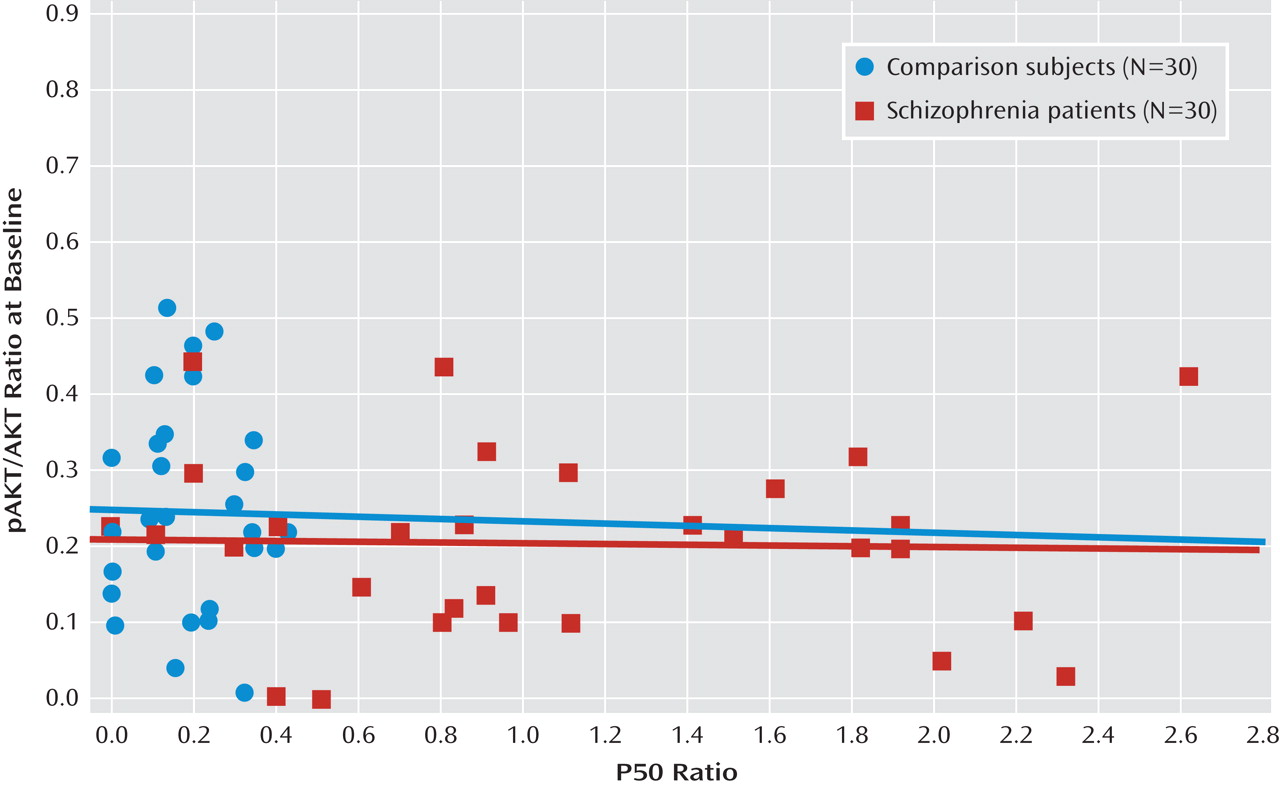
Figure 2 depicts the relationship between P50 suppression and AKT phosphorylation after neuregulin 1 stimulation. Higher neuregulin 1-induced phosphorylated AKT to total AKT ratios were associated with stronger P50 suppression in comparison subjects (r
s=–0.50, p<0.01) and in patients with schizophrenia (r
s=–0.70, p<0.0001). In the baseline condition, there was no such relationship in comparison subjects (r
s=–0.03) or in patients with schizophrenia (r
s=–0.09) (
Figure 3). The P50 amplitudes did not show significant correlations with AKT phosphorylation in either group of participants (–0.2< r
s<0.2).
Discussion
The results of this study indicate that never-medicated, first-episode patients with schizophrenia exhibit diminished P50 suppression, which is associated with reduced neuregulin 1-induced AKT phosphorylation in peripheral lymphoblasts. This is consistent with the hypothesis that neuregulin 1-related signals may be important in the regulation of neuronal circuits participating in sensory gating.
Recently, two meta-analyses questioned the validity of P50 suppression as an endophenotype of schizophrenia (
6,
7), and one study did not find impaired P50 suppression in medicated first-episode patients with schizophrenia (
8). Both meta-analyses emphasized the importance of methodological standards in order to obtain replicable and less heterogeneous results across different laboratories. de Wilde et al. (
6) concluded that differences in methodology and lack of reported demographics make it more difficult to compare results across studies from different investigators. Although antipsychotic medication did not appear as a confounding variable in meta-analyses, there is some evidence that the so-called second-generation antipsychotics may normalize P50 suppression (
32). de Wilde et al. (
6) also found that there are significant differences between studies conducted at the University of Colorado and those done in other laboratories. For example, the magnitude of P50 suppression shows a large variability across different laboratories. The mean comparison group P50 ratios reported by the Colorado group are significantly lower than the mean for most other comparison groups from different laboratories (
7). The P50 ratio in healthy subjects is around 0.4–0.5, whereas in our study it was 0.18. This smaller ratio is consistent with the results of studies using the Colorado protocol (
7). The discrepancies may be due to differences in methodology, including sound intensity, filters, participants' position, smoking status, and demographic characteristics. In the present study, we adopted the protocol of the research group at the University of Colorado and were able to replicate the P50 suppression deficit in a nonmedicated, first-episode schizophrenia group. The majority of the patients were nonsmokers, and there were no significant differences between smokers and nonsmokers after nicotine withdrawal. However, the number of smokers was small and the statistical power was limited. Therefore, caution is warranted in the interpretation of these data. Altogether, these results suggest that by the application of methodological standards, the P50 suppression paradigm is suitable for the detection of sensory gating deficits even in young patients with schizophrenia at the beginning of the illness. This is consistent with the data from a German (
33) and a Turkish group (
34).
The new finding of this study was that we demonstrated a significant correlation between P50 suppression and neuregulin 1-induced AKT phosphorylation in the peripheral lymphoblasts of patients and comparison subjects. These results may indicate that less efficient intracellular signaling is associated with poorer sensory gating, which is in accordance with animal models revealing interactions between neuregulin 1 and cholinergic neurotransmission (
19,
20). Genetic studies indicated that various polymorphisms of the neuregulin 1 gene are linked to schizophrenia, although the evidence is not conclusive (
35). Postmortem studies revealed an altered expression of neuregulin 1 gene in the brain of patients with schizophrenia, but these are not equivocally linked to functional polymorphisms, and both increased and decreased expressions of different isoforms have been reported (
36). Some genetic variants of neuregulin 1 are related to decreased brain activation during cognitive tasks and to increased risk of psychosis conversion in people who are at high risk for psychosis (
37,
38). Sei et al. (
13) also demonstrated decreased neuregulin 1-induced AKT phosphorylation in the peripheral lymphoblasts in schizophrenia, whereas baseline AKT phosphorylation was less altered, similar to the results of Ide et al. (
39) and to our previous observations (
40).
The most important limitation of the present study is that the correlation between P50 suppression and AKT phosphorylation in lymphoblasts is indirect and complex, and its relevance to information processing in the CNS must be proven. Nevertheless, this association is supported by animal models and by the interaction between neuregulin 1-related signaling processes and cholinergic neurotransmission in sensory gating (
19,
20). In addition, the correlation between AKT phosphorylation and P50 suppression was confined to the neuregulin 1-induced condition and was absent in the baseline condition. This finding supports the biological relevance of the results. Another limitation is that unaffected biological relatives were not included, and therefore our data are not conclusive regarding the endophenotype status of P50. Finally, neuronal oscillations with high heritability may be a better endophenotype of sensory gating than evoked potentials (
41).
In summary, we demonstrated a significant association between electrophysiological and peripheral molecular markers of schizophrenia. These findings may help elucidate the pathophysiology of the illness and may facilitate the identification of new targets for drug treatment.
Acknowledgments
The authors thank John Bowen and Imre Kiss for their help in data collection and analysis and the VitaTrade Kft for technical assistance.