Bulimia nervosa typically begins in adolescence and primarily affects women. It is characterized by recurrent episodes of binge eating (consumption of an excessive amount of food) followed by self-induced vomiting or another compensatory behavior to avoid weight gain.
An extreme sense of loss of control accompanies these binge eating episodes (
1,
2). Mood disturbances and behavioral dyscontrol that includes impulsive, aggressive, and compulsory behaviors are also common in persons with bulimia nervosa, suggesting the presence of pervasive difficulties in behavioral self-regulation (
2).
Our previous functional neuroimaging findings in adult female patients with bulimia nervosa suggest that frontostriatal systems that are not engaged may contribute to impaired self-regulation in this population (
3). Women with bulimia nervosa responded more impulsively, making more errors than healthy comparison subjects on the Simon spatial incompatibility task, which requires participants to ignore a task-irrelevant feature of a stimulus (e.g., the side of the screen on which an arrow appears) when it conflicts with a more task-relevant feature (e.g., the direction that the arrow is pointing). Therefore, self-regulatory control is required to respond correctly on conflict-laden incongruent trials. During correct responses on these trials, frontostriatal circuits—including the inferolateral prefrontal cortex, inferior frontal gyrus, anterior cingulate cortex, putamen, and caudate—failed to activate to the same extent in women with bulimia nervosa as in healthy comparison subjects. In addition, these regions were engaged the least among individuals with the most severe bulimic symptoms, who performed most poorly on the task. These findings suggest that the inability of fronto-striatal systems to engage appropriately likely contributed to the origins of their binge eating and other impulsive behaviors in women with bulimia nervosa. However, we could not know when dysfunction in frontostriatal systems arose or how it characterized the early disease process, disentangled from the effects of chronic illness.
In the present article, we report on an event-related functional magnetic resonance imaging (fMRI) study in which we used a modified version of the Simon task to investigate the neural substrates of self-regulatory control in female adolescents with and without bulimia nervosa. This shorter task consisted of three rather than 10 scan runs, making it more suitable for younger participants. In addition, congruent and incongruent stimuli appeared equally as often, thereby eliminating potential oddball effects associated with the infrequent presentation of incongruent stimuli and reducing the priming effects associated with long repeated trials of congruent stimuli. Because this modified task is easier than the standard version used in our prior study (
3), we suspected that the bulimia nervosa and healthy comparison groups would perform with similar reaction times and with few response errors on both incongruent and congruent trials. However, we hypothesized that similar to our findings from adults with the disorder, frontostriatal regions in adolescents with bulimia nervosa would not activate to the same extent as in healthy adolescents during correct responses to incongruent stimuli, reflecting an inability of these circuits to engage in the service of self-regulation and conflict resolution. Behavioral studies of the Simon task suggest that healthy individuals experience more cognitive conflict (i.e., they respond more slowly) in response to incongruent stimuli that are preceded by congruent stimuli than in response to incongruent stimuli that are preceded by incongruent stimuli (
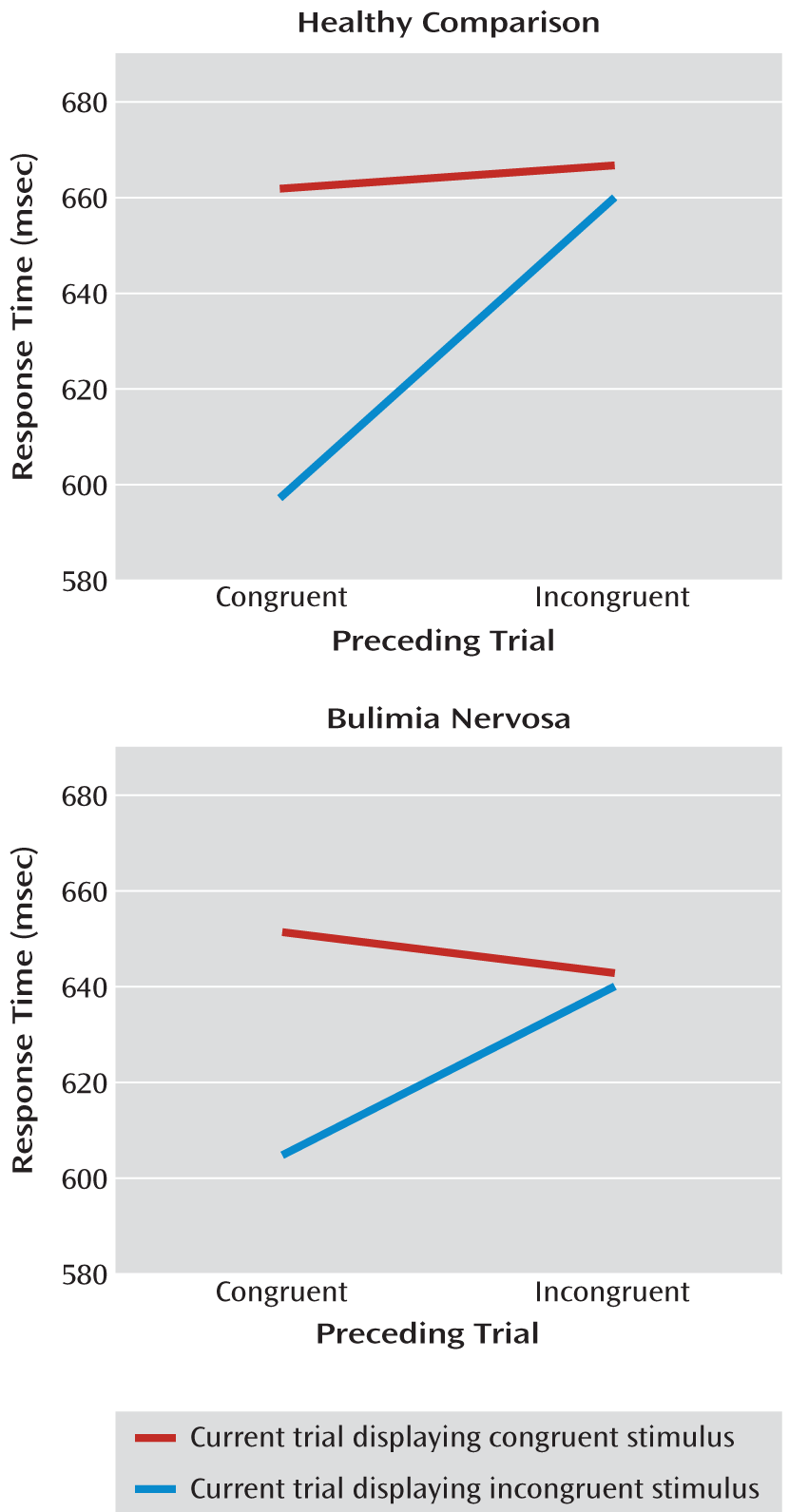
4,
5). Conflict in a preceding incongruent trial tends to enhance self-regulatory control in a current incongruent trial, facilitating processing and making responses faster and more accurate. Thus, the preceding stimulus context likely contributes to greater engagement of frontostriatal regions needed to respond correctly in postcongruent conflict trials (
6,
7). We therefore suspected that the preceding stimulus context would contribute to group differences in brain activation associated with correct responses to incongruent stimuli relative to congruent stimuli.
Method
Participants
Female adolescents with bulimia nervosa were recruited through flyers posted in the local community and advertisements posted on Internet sites (e.g., craigslist.com and eating disorder-specific websites) as well as through the New York State Psychiatric Institute Eating Disorders Clinic, where some were receiving outpatient treatment. Individuals receiving medication treatment were not excluded. Healthy comparison female adolescents were also recruited through flyers and Internet advertisements. Participants with bulimia nervosa and healthy comparison subjects were group-matched by age and body mass index. The Institutional Review Board of the New York State Psychiatric Institute approved this study, and all participants gave informed consent prior to study entry.
The details regarding exclusion criteria, clinical and behavioral assessments, MRI pulse sequence, image processing, behavioral analysis, and exploratory image analyses are described in the data supplement accompanying the online version of this article.
fMRI Paradigm
Stimuli were presented through nonmagnetic goggles (Resonance Technologies, Inc., Los Angeles) using E-Prime software (Psychology Software Tools, Inc., Sharpsburg, Pa.). A series of white arrows pointing either left or right was displayed against a black background either to the left or to the right of a white gaze fixation crosshair positioned at midline. Stimuli subtended 1 vertical degree and 3.92 horizontal degrees of the visual field. Stimuli were congruent (pointing in the same direction as their position on the screen), incongruent (pointing in a direction opposite their position on the screen), or blank (a crosshair positioned at midline).
Participants were instructed to respond quickly to the direction of the arrow by pressing a button on a response box using the index finger of their right hand for a left-pointing arrow and the middle finger of that hand for a right-pointing arrow. The button press recorded responses and reaction times for each trial containing congruent or incongruent stimuli. Stimulus duration was 1,300 msec, with a jittered interstimulus interval (mean=5,352 msec [SD=842]; range=4,009–6,857 msec). Each scan run contained 55 stimuli (duration: 5 minutes, 7 seconds), with 22 congruent stimuli (11 left-pointing arrows presented to the left of midline and 11 right-pointing arrows presented to the right of midline), 22 incongruent stimuli (11 left-pointing arrows presented to the right of midline and 11 right-pointing arrows presented to the left of midline), and 11 blank stimuli (longer periods of fixation). These stimuli were arranged and presented in a pseudorandom order. Each experiment contained three scan runs, totaling 66 congruent and 66 incongruent stimuli.
Image Analysis
First-level parametric analyses were performed individually for each participant using a modified version of the general linear model function in SPM8 (Wellcome Department of Imaging Neuroscience, London [
http://www.fil.ion.ucl.ac.uk/spm/]). Preprocessed blood-oxygen-level-dependent (BOLD) time series data at each voxel, concatenated from all three scan runs of the Simon task (420 volumes), were modeled using a general linear model, with the following five predictors corresponding to each of the different trial types (a total of 15 independent variables): 1) congruent correct trials, 2) incongruent correct trials, 3) blank trials, 4) fixation trials, and 5) all incorrect trials (either congruent or incongruent). All of these events were then convolved with the canonical hemodynamic response function (
8). For each participant, least-squares regression was used to estimate parameters for each of the 15 independent variables. The parameter estimates for the three scan runs were summed to produce an incongruent correct versus congruent correct contrast for each participant that was used to assess brain activity during the engagement of self-regulatory control. Additional planned contrasts (incongruent correct versus blank, incongruent correct versus fixation, congruent correct versus blank, and congruent correct versus fixation) were also included to establish the direction of BOLD signal associated with congruent and incongruent events relative to baseline events (blank or fixation trials). Because of the limited number of incorrect trials, contrasts involving these trials were not assessed.
Group differences in brain activations were tested using a second-level Bayesian analysis (
9–
11). We report voxels that were identified as having a posterior probability of 97.5%, using a p-value threshold <0.025 and a cluster filter of at least 25 adjacent pixels, a conjoint requirement that, based on an approximation formula (
12), yields a conservative effective p value that is <0.000005. The combined application of a statistical threshold and cluster filter reduces substantially the false positive identification of activated pixels at any given threshold (
13).
Hypothesis Testing
We tested whether female adolescents with and without bulimia nervosa differed in brain activity during correct responses for incongruent trials relative to correct responses for congruent trials. A Bayesian analysis was conducted to identify significant group differences in frontostriatal regions. Further details of our exploratory hypotheses are provided in the data supplement.
Discussion
Adolescents with and without bulimia nervosa performed similarly on a rapid version of the Simon task. Both groups responded more slowly to incongruent stimuli relative to congruent stimuli and made few errors on the task (
Figure 6). Nevertheless, participants with bulimia nervosa did not demonstrate the same magnitude of neural activity as healthy comparison subjects in the fronto-striatal systems known to subserve self-regulatory control, including the right inferior frontal gyrus, dorsolateral prefrontal cortex, and putamen. These findings are consistent with our prior fMRI findings in adult female patients (
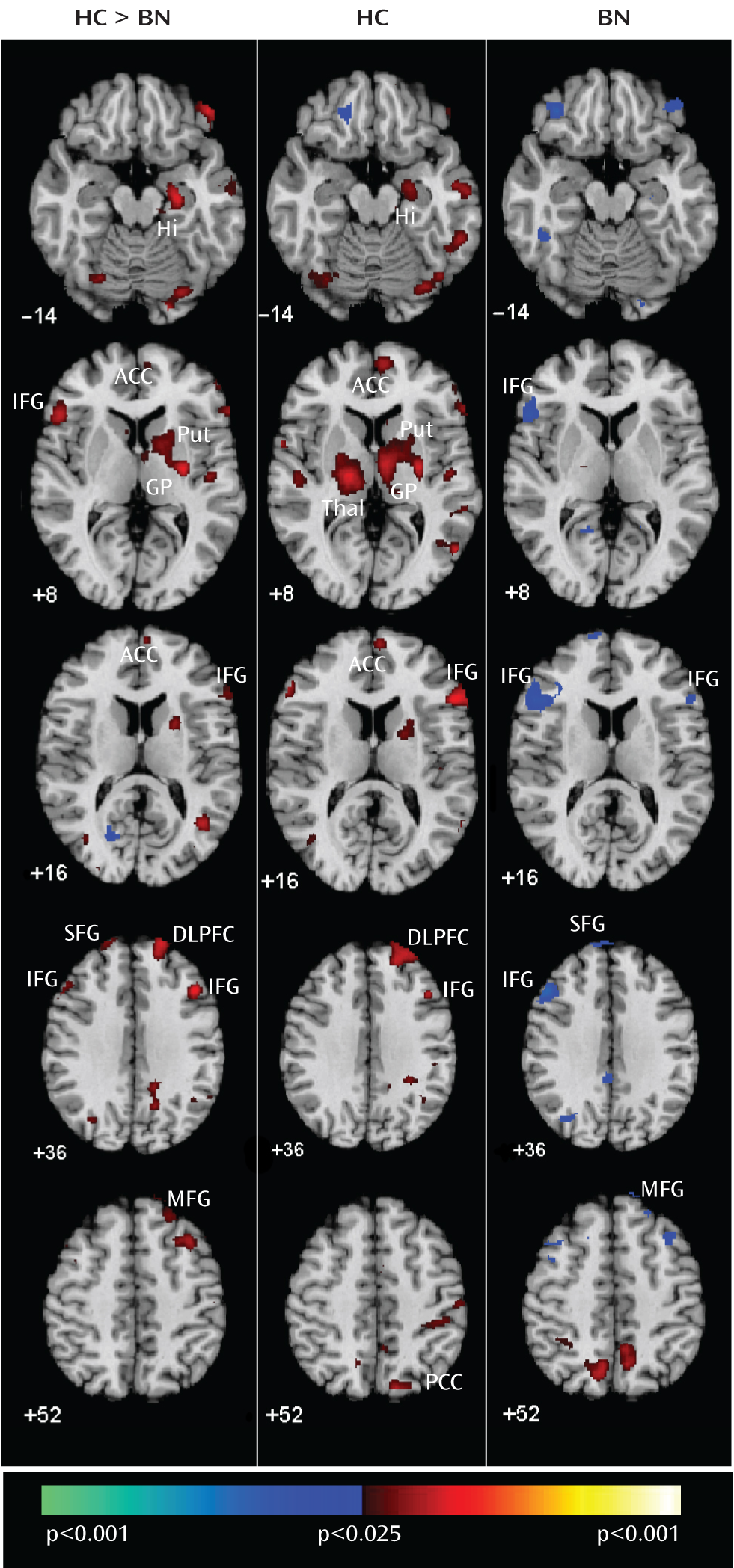
3). We previously speculated that the inability of these systems to engage appropriately in women with bulimia nervosa may exacerbate and interfere with their ability to resolve their conflicting desires to consume fattening foods and avoid weight gain. This inability may also contribute to their difficulty regulating binge-type eating and other impulsive behaviors. Our findings in adolescents with bulimia nervosa suggest that the inability to engage frontostriatal systems to resolve conflict and regulate behaviors may arise early in the course of the illness.
When responding correctly to incongruent stimuli compared with congruent stimuli, deactivation was seen in the left inferior frontal gyrus as well as a neural system encompassing the posterior cingulate cortex and superior frontal gyrus in adolescents with bulimia nervosa. The posterior cingulate cortex is a major node of the default-mode network of brain areas that activate when individuals are engaged in internally driven thoughts and decrease in activity when attention is directed to a task (
14). The superior frontal gyrus is also part of this network (
15), and recent findings suggest that the posterior cingulate cortex and superior frontal gyrus are functionally connected to the medial and inferior frontal gyri (
16). Deactivation of all of these brain areas in response to the presentation of both congruent and incongruent stimuli was observed in adolescents with bulimia nervosa, perhaps suggesting that they may have been attending to internally driven thoughts, such as thoughts about eating or body image that preoccupy individuals with eating disorders.
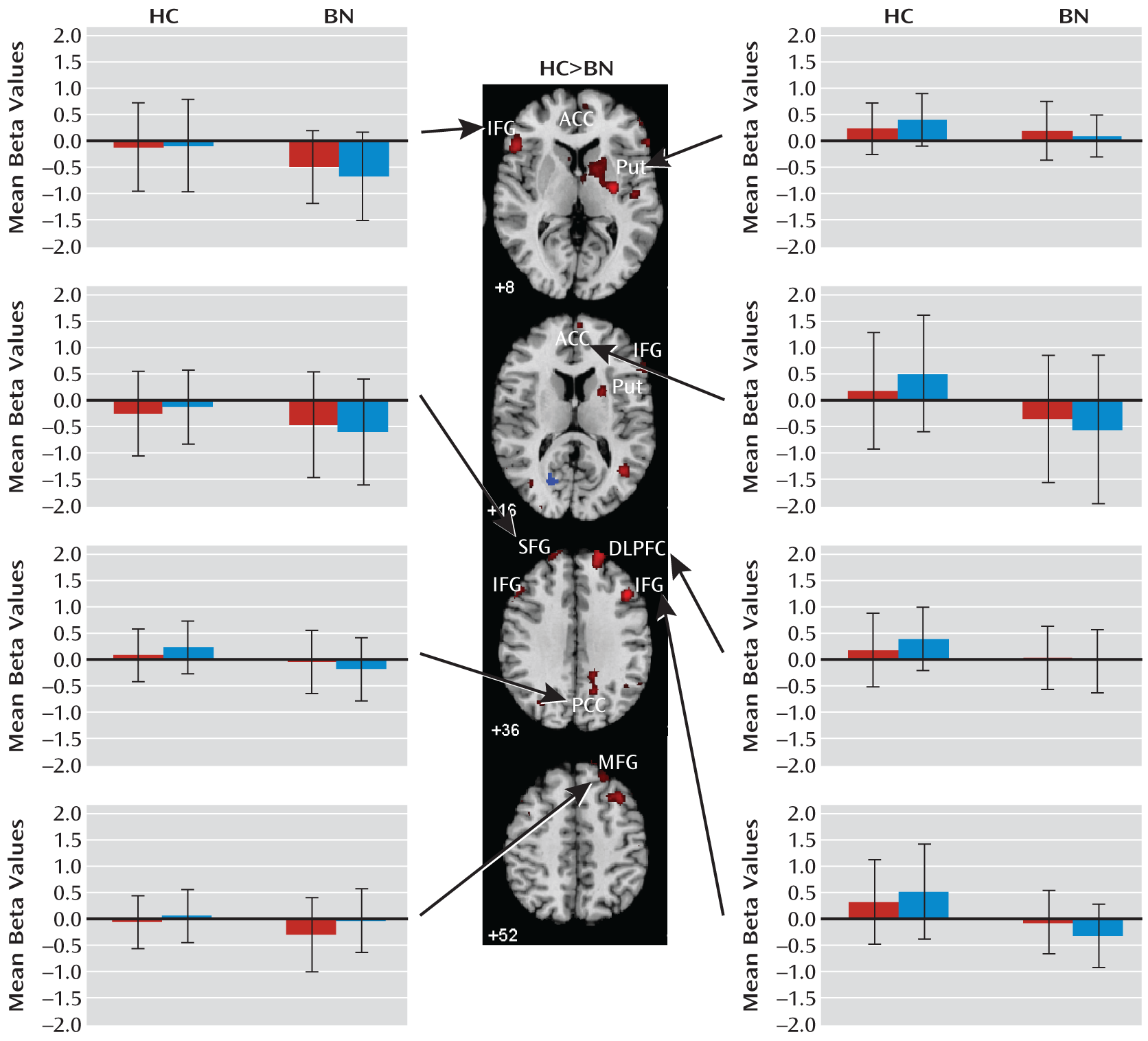
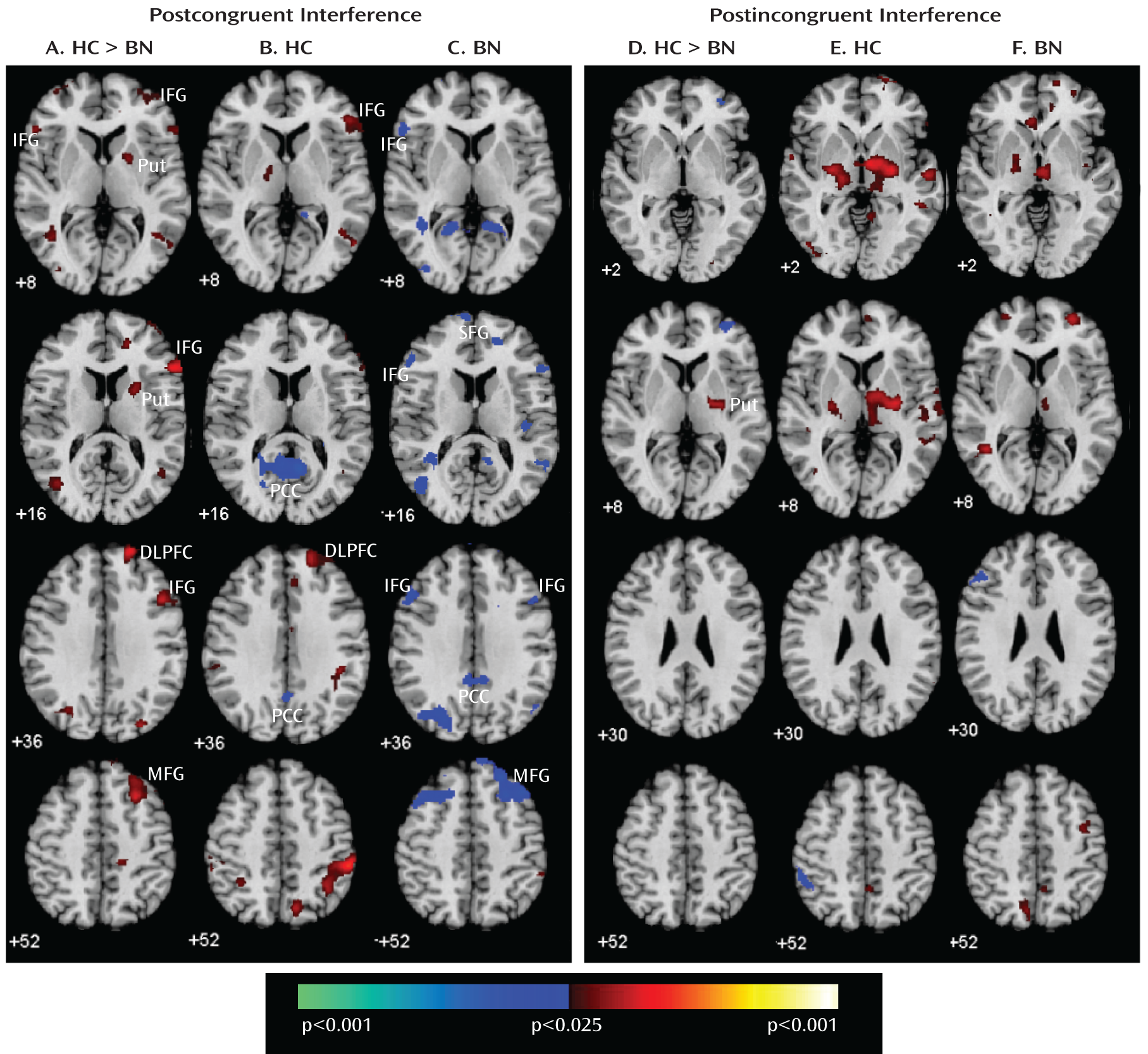
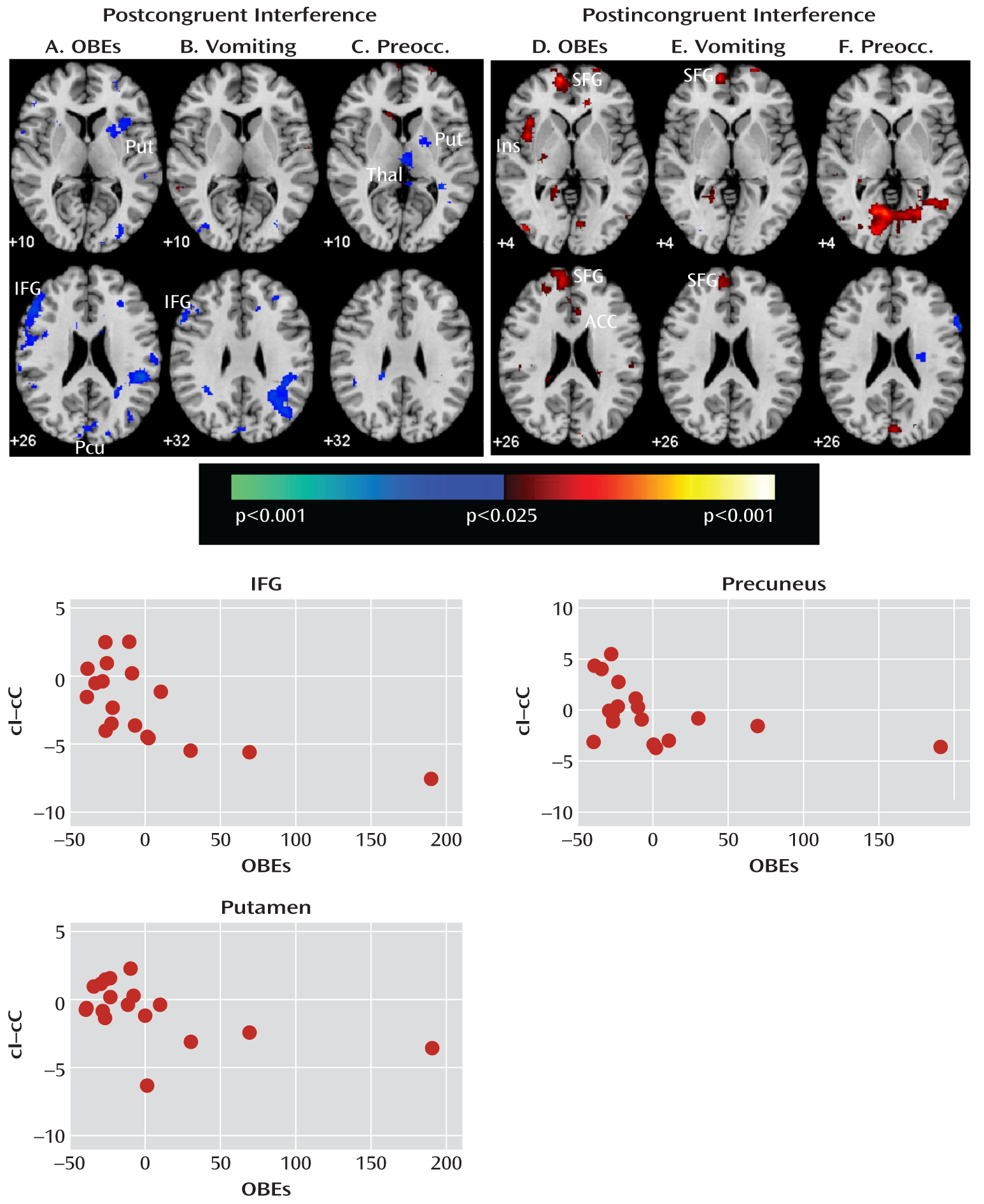
Our analyses suggest that functional abnormalities in frontostriatal and default-mode systems in female adolescents with bulimia nervosa derived from altered processing of cognitive interference, which depended on the presence or absence of antecedent conflict, and were particularly driven by abnormal processing of cognitive interference that followed congruent stimuli, when interference effects were greatest in both the bulimia nervosa and healthy comparison groups (postcongruent interference: incongruent preceded by congruent-congruent preceded by congruent contrast [
Table 4,
Figure 1]). In this contrast, deactivation in the inferior and superior prefrontal cortices was seen in female adolescents with bulimia nervosa, whereas activation in the right frontostriatal regions—including the inferior and dorsal prefrontal cortices and putamen—was seen in healthy female adolescents (
Figure 4). Deactivation in the posterior cingulate cortex in response to postcongruent interference was found in both groups, but deactivation in the posterior cingulate cortex and medial and superior frontal gyri in response to both congruent and incongruent stimuli was seen only in the bulimia nervosa group (
Figure 3). In addition, greater deactivation of these cortical regions was associated with more severe symptoms, suggesting that cortical deactivation in the bulimia nervosa group may have represented the intrusion of self-referential and illness-related thoughts when processing interference in the absence of prior conflict. In the bulimia nervosa group, deactivation of the superior frontal gyrus and posterior cingulate cortex in response to congruent stimuli that were preceded by incongruent stimuli relative to congruent stimuli (congruent preceded by incongruent-congruent preceded by congruent contrast [see Figure 2 in the data supplement]) suggests that deactivation of these default-mode regions was specific to the anteceding conflict-laden context rather than current conflict. This finding further emphasizes the contextual origins of the group differences in this network.
The number of objective bulimic and vomiting episodes correlated inversely with activity associated with postcongruent interference in the left inferior frontal gyrus, a region that was deactivated in response to incongruent stimuli in the bulimia nervosa group. The number of bulimic episodes and ratings of preoccupation with body shape and weight also correlated inversely with postcongruent interference in the right putamen. Thus, in the bulimia nervosa group, deactivation in the inferior frontal gyrus was seen the most and activation in the subcortical portions of frontostriatal circuits was seen the least among the most symptomatic individuals when responding to postcongruent interference, consistent with our prior findings that frontostriatal activation correlated with symptom severity in adult female patients with bulimia nervosa (
3). The task used in our previous study consisted of more congruent trials than incongruent trials, and thus the analysis of interference effects (incongruent versus congruent comparison) in that study was most similar to the postcongruent interference contrast in the present study.
Group differences in subcortical activations were driven by the differential processing of cognitive interference following incongruent stimuli (incongruent preceded by incongruent-congruent preceded by incongruent contrast [
Table 4]), which is the contrast that produced the least interference in both groups. Activation of frontostriatal regions in this condition was observed in both groups (i.e., postincongruent interference), but activation of the subcortical portions of these circuits was seen in the healthy comparison group more than the bulimia nervosa group, suggesting that the healthy subjects relied more on an automatic response tendency based within the striatum (
17) when responding to repeated conflict over successive trials and that this adaptive habitual responding may be deficient in adolescents with bulimia nervosa. These differential patterns of brain activation when resolving cognitive interference following congruent or incongruent stimuli, respectively, contributed to the overall group differences in our primary analyses of cortical and subcortical brain activation in processing cognitive interference that we detected for analyses of all trials, regardless of the presence or absence of antecedent conflict (
Figure 2).
Our findings suggest that frontostriatal functioning is impaired in bulimia nervosa early in the course of illness. When engaging the self-regulatory control processes necessary to resolve cognitive interference and respond correctly in incongruent trials, adolescents with bulimia nervosa did not display normal patterns of activation in frontostriatal circuits or deactivation in default-mode systems. Abnormal processing of the antecedent stimulus context conditioned their brain responses to conflict differently, particularly in frontal regions when preceding stimuli were congruent and conflict-free. Given that the bulimia nervosa and healthy comparison groups performed similarly on the task and that our analyses were limited to activations associated with correct responses, these differences in brain activation did not derive from performance differences and instead represented functional disturbances in neural systems that subserve self-regulation and the resolution of interference. The Simon task used in this study differed from the longer version of the task used in our prior study of adults with bulimia nervosa (
3). Congruent and incongruent stimuli appeared equally as often using the shorter version, thereby making it easier to inhibit the prepotent response tendency, and as hypothesized, the groups performed similarly on the task. In addition, context effects could be assessed with this task design, contributing to our additional finding that abnormal contextual processing likely affects the neural processing of conflict in adolescents with bulimia nervosa. These findings of impaired frontostriatal functioning may inform clinical practice, suggesting that strategies for improving self-regulatory control and conflict resolution through cognitive remediation therapy may be a promising treatment for bulimia nervosa in adolescents.
A limitation of this study was the absence of a comparison group consisting of impulsive individuals with normal weight and eating behaviors, which would permit assessment of the specificity of frontostriatal abnormalities in adolescents with bulimia nervosa. Other limitations were the modest sample size and presence of comorbid illnesses in participants with bulimia nervosa, some of whom were receiving medication treatment. In addition, participants had varying symptom severities, and individuals with full- and partial-syndrome bulimia were included. However, given that subthreshold eating disorders are most prevalent among adolescents (
18), our sample represented well the general population of adolescents with bulimia nervosa. Moreover, our inclusion criteria were consistent with the lower and more developmentally sensitive thresholds that are more appropriate for adolescents and that have been proposed for DSM-5 (
19). Nevertheless, future studies should include larger samples of adolescents with bulimia nervosa and assess changes in the functioning of frontostriatal and default-mode systems in the same participants over time to understand whether disturbances in these systems may contribute to the development and progression of the illness.