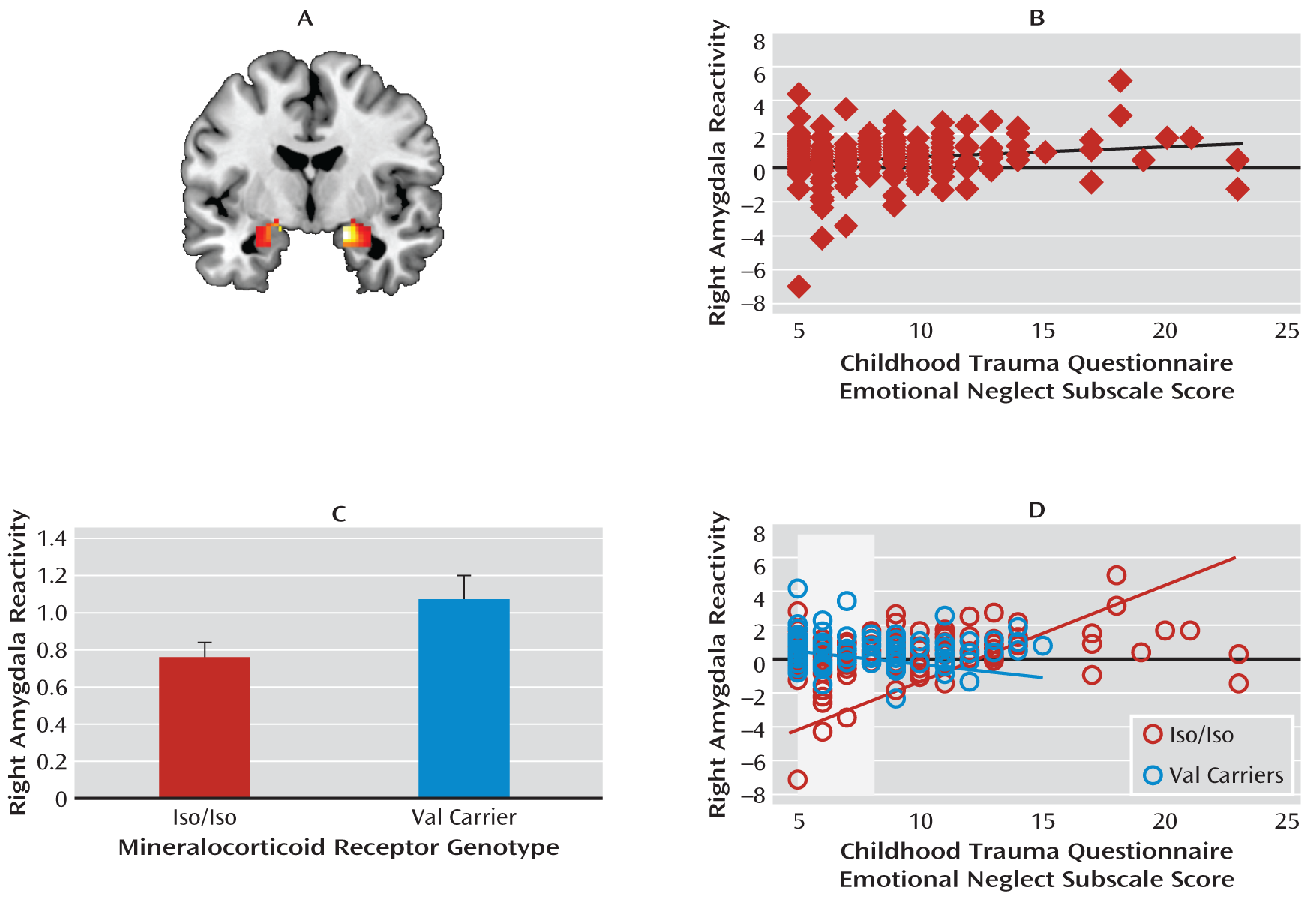
We examined how a functional genetic polymorphism affecting HPA axis function and a history of childhood emotional neglect independently and interactively influence amygdala reactivity to threatening stimuli. Three primary findings emerged. First, extending recent research linking extreme forms of emotional neglect (e.g., institutional rearing) to heightened threat-related amygdala reactivity (
4,
6), we observed a similar pattern with self-reported emotional neglect in a community sample. Second, we found that the val allele was associated with heightened threat-related amygdala reactivity (however, this effect fell short of significance when we included ancestry-informative principal components but remained significant in the Caucasian-only subsample; see the online data supplement). This effect is consistent with evidence that the val allele of a common mineralocorticoid receptor iso/val polymorphism confers heightened neuroendocrine and autonomic responses to stressors (
20,
22,
24). Third, we observed an interaction between the mineralocorticoid receptor genotype and a history of childhood emotional neglect such that a positive association between emotional neglect and threat-related amygdala reactivity was observed only in iso homozygotes, and val carriers had heightened amygdala reactivity relative to iso homozygotes only at relatively low levels of previous childhood emotional neglect.
Childhood Emotional Neglect
Early life stress is one of the strongest predictors of developing emotional, cognitive, and behavioral difficulties (
37). However, the mechanisms by which early adversity is associated with such difficulties are poorly understood. This study extends recent findings that extreme forms of childhood emotional neglect are associated with increased amygdala volume (
7) and threat-related reactivity (
4,
6). Here we show that self-reported history of emotional neglect in children from a community sample is similarly associated with heightened amygdala reactivity, providing initial evidence that milder forms of neglect are also associated with biases in neural function related to an increased risk for psychopathology.
Animal research has shown that a host of early life stressors such as maternal separation are associated with disrupted diurnal HPA axis activity and increased HPA axis and autonomic excitability (
3). This evidence is buttressed by limited human research showing that day care (a putative proxy for maternal separation) is associated with similar, albeit less pronounced, differences (
38). Moreover, animal models have linked early stressors with increased dendritic branching, the expression of corticotropin-releasing hormone, and neuronal excitability in the amygdala, which are correlated with HPA axis activity and anxiety (
3). Consistent with these findings, elevated endogenous cortisol is associated with potentiated amygdala reactivity to emotional facial expressions like those used in the present study (
9,
10).
At the cellular level, animal research suggests that stress may potentiate amygdala reactivity to threat by increasing calcium influx in basolateral amygdala neurons, thus increasing excitatory and reducing inhibitory neurotransmission (
39). Human studies show that pharmacologically induced elevations of cortisol and norepinephrine together potentiate amygdala reactivity to threatening faces (
40). However, pharmacologically administered hydrocortisone alone blunts amygdala reactivity, likely because of glucocorticoid-mediated negative feedback in the context of no other HPA axis activation (
40,
41). We speculate that changes in HPA axis function as well as amygdala cytoarchitecture in people with a history of childhood emotional neglect may promote heightened amygdala reactivity to threat. This heightened reactivity may translate to increased vigilance and lay the groundwork for the development of stress-related psychopathologies such as mood and anxiety disorders. Consistent with this speculation, the association between stress and anxiety-related behavior in animals is abolished with the antagonism of excitatory
N-methyl-
d-aspartic acid neurotransmission in the amygdala (
42).
Mineralocorticoid Receptor Genotype Effects and Moderation of Childhood Emotional Neglect
Mineralocorticoid receptor val carriers had heightened amygdala reactivity relative to iso homozygotes. Previous studies have linked the val allele to the loss of cortisol-related mineralocorticoid receptor function and heightened stress reactivity (
20,
21,
24; but also see
25). Endogenous cortisol is associated with elevated amygdala reactivity (
9,
10), and overexpression of the mineralocorticoid receptor in the amygdala reduces corticosterone release in rodents (
19). The relatively potentiated amygdala reactivity in val carriers observed in our study may reflect reduced cortisol-related mineralocorticoid receptor function and heightened basal cortisol levels. The val allele may confer a constitutional profile similar to that observed in maltreated individuals (e.g., heightened basal HPA axis function, blunted cortisol upon awakening, and heightened stress-related endocrine and autonomic responses), and thus similar stress system mechanisms may underlie the relationship between the val allele and heightened amygdala reactivity to threat.
Childhood emotional neglect was positively associated with amygdala reactivity only in iso homozygotes, and genotype groups differed from one another only at relatively low levels of emotional neglect, such that val carriers had elevated amygdala reactivity relative to iso homozygotes. We speculate that the nature of this gene-by-environment interaction may reflect shifts in the neuroendocrine and autonomic profiles of val allele carriers. It is possible that relatively elevated activity of the HPA axis places val carriers closer to a physiological ceiling and thus makes them relatively insensitive to further modulation of amygdala reactivity by previous emotional neglect. The finding of generally greater amygdala reactivity in val carriers relative to iso homozygotes is consistent with this speculation. Additionally, threat-related amygdala reactivity in iso homozygotes may be more sensitive to environmental conditions, for better or worse (
43).
The overall effect of task was associated with left and right amygdala reactivity, consistent with a recent meta-analysis (
44). However, the emotional neglect and mineralocorticoid receptor genotype findings were lateralized to the right. Evidence suggests that the right amygdala subserves quick and perhaps automatic stimulus detection while the left amygdala provides a more sustained evaluative response (
44). This interpretation is consistent with the role of the mineralocorticoid receptor in the initiation of stress response as well as the heightened HPA axis activation conferred by the val allele and observed in individuals with a history of childhood emotional neglect. Accordingly, early emotional neglect and mineralocorticoid receptor genotype may influence the initial phase of threat detection, which may lead to exaggerated responsiveness to mildly threatening and even neutral or positive events.
The amygdala can be divided into anatomically defined ventral (containing the basolateral complex) and dorsal (containing the central nucleus and sublenticular extended amygdala) subregions. A host of animal studies have evaluated the effects of stress and mineralocorticoid receptor expression specifically in the basolateral complex of the amygdala (
19,
39). Our exploratory analyses of ventral and dorsal amygdala subregions (see the online data supplement) showed no evidence that either structure was specifically involved in the effects we found in our study. Rather, we found a general effect on activation extending across both the ventral and dorsal subregions. This may reflect a glutamatergic drive of ventral neurons on dorsal neurons, resulting in the generally increased activity seen in the BOLD fMRI signal from the entire amygdala. Additionally, the trend-level effect in the right dorsal but not ventral amygdala may reflect the specific connections between the nuclei of the dorsal subregion (e.g., the central nucleus) and HPA axis targets (e.g., the paraventricular nucleus), which would be particularly influenced by differences in mineralocorticoid receptor signaling.
Emotional neglect alone and in interaction with mineralocorticoid receptor genotype was associated with right amygdala reactivity. However, none of the other Childhood Trauma Questionnaire subscales (emotional abuse, physical neglect, physical abuse, and sexual abuse) were associated with significant effects. As shown in
Table 1, emotional neglect was the most endorsed subscale and contained the most variability. Virtually no sexual abuse, physical neglect, or physical abuse was reported in this sample, which may account for the lack of associations with amygdala reactivity and these variables. Moreover, the specificity of this association is not entirely surprising. Previous research on childhood adversity in both animals (e.g., maternal separation) and extreme phenotypes in humans (e.g., institutional rearing) captures experiences best approximated by emotional neglect as opposed to other forms of maltreatment assessed by the Childhood Trauma Questionnaire (
3,
4,
6,
7). We speculate that because emotional neglect leaves individuals with fewer support resources to buffer the negative consequences of stress and threat, it may be particularly likely to result in greater and perhaps more generalized sensitivity to threat. This speculation is consistent with data indicating that emotional neglect predicts trauma exposure and contributes to depression and anxiety even when controlling for physical and sexual maltreatment (
45). It is further reflected in recent findings that perceived social support may buffer against the expression of anxiety as a function of amygdala reactivity (
34).
This is one of the first imaging gene-by-environment interaction studies. It provides an example of how incorporating the environment into imaging genetics research can clarify the relationships between genes and brain function (
46). Moreover, it has become increasingly evident in imaging genetics that common single genetic polymorphisms are likely to have small effects, if any, on brain function or behavior (
2). By incorporating the environment into such models, we may gain traction in demonstrating relationships between these small effects and behaviorally relevant brain function (
46). While our entire model accounted for 7% of the variance in threat-related amygdala reactivity, the genotype-by-emotional neglect interaction only accounted for 1.4% of the variance in amygdala reactivity after controlling for main effects of genotype and emotional neglect. This is a relatively small amount of independent variance; however, when viewed in the context of the overall model it still demonstrates significant predictive utility for questions of basic science and, potentially, even those related to clinical issues (e.g., novel molecular targets). For example, studies reporting associations between amygdala reactivity and 5-HTTLPR genotype have accounted for similar percentages of variance (
47); given that those findings have been shown to have clinical relevance (
48), the small effects reported here may as well. Moreover, it is possible that even small differences in how the amygdala processes information result in a cascade of neural, hormonal, and physiological changes that affect behavior and clinical risk; such small effects may be particularly pertinent in the context of cellular and nuclear receptors in the HPA axis, which can act as transcription factors to widely influence gene expression (
49).
However, the small amount of additional variance also demonstrates the need for other techniques, such as the use of biologically informed multilocus genetic profiles, to better represent underlying molecular differences (
50). A genetic profile representing variability in multiple levels of the HPA axis might offer particular utility; for example, polymorphisms that are known to affect stress response (e.g., rs5522) as well as those that have been associated with impaired negative feedback of the HPA axis (e.g., FKBP5 polymorphisms [
51]) could be combined into a single genetic profile score that better represents HPA axis function.
Our study is not without limitations. First, while we speculated on the relationship between HPA axis function and amygdala reactivity in the context of a history of childhood emotional neglect and mineralocorticoid receptor genotype, we did not collect neuroendocrine measures (e.g., cortisol) to directly test whether such differences might mediate this relationship. Second, the assessment of emotional neglect relied on retrospective self-report. Given that such retrospective memory may be influenced by other factors that are also associated with amygdala reactivity (e.g., neuroticism), it will be important for future research to replicate these findings with more refined assessments (e.g., public records and interviews). The Childhood Trauma Questionnaire nevertheless possesses excellent reliability and validity relative to other measures (e.g., clinician-rated abuse from interview) (
30). Moreover, our results nicely complement animal research and human research in extreme environments (e.g., institutional rearing), lending credence to the findings reported here (
4–
9). It will be important for researchers to examine whether associations between mineralocorticoid receptor genotype, emotional neglect, and amygdala reactivity predict individual differences in behavior and psychopathology. Prospective research that follows individuals into peak periods of psychopathology development, as the Teen Alcohol Outcomes Study is designed to do, will be particularly valuable (
52).
Limitations notwithstanding, this study provides important evidence demonstrating that a common functional polymorphism associated with variability in the responsiveness of the HPA axis moderates the impact of childhood emotional neglect on threat-related amygdala reactivity. If replicated in additional samples, the neural effects described here may represent a biological mechanism mediating stress responsivity and risk for psychopathology associated with this polymorphism.