At least 8% of all pregnancies in the United States include treatment with antidepressants (
1), primarily for anxiety and mood disorders. Yet, the use of antidepressants remains controversial. While there have been no randomized controlled trials of antidepressants in pregnant women, cessation of ongoing treatment is associated with psychiatric illness relapse (
2), and risks and benefits for the pregnant woman are considered to be similar to those found in nonpregnant populations (
3). Concerns arise because of a lack of knowledge about the potential effect of antidepressant exposure on the developing fetus.
In utero exposure to elevated maternal stress is generally accepted as having lifelong ramifications. Children with a history of in utero exposure to maternal anxiety, depression, or other forms of prenatal stress have an elevated risk for a variety of neuropsychiatric conditions, including general psychopathology such as internalizing and externalizing disorders (
4), specific psychopathological symptoms such as those found in attention deficit hyperactivity disorder, anxiety, and depression (
5–
9), and generally lower neurocognitive performance, particularly in areas of attention and memory (
10–
12). The impact of maternal stress on offspring outcome has been demonstrated, even when controlling for confounding factors such as tobacco exposure, socioeconomic status, and later maternal psychopathology. Epidemiological studies, which often focus on a specific diagnosis, have found that in utero exposure to elevated levels of maternal stress is associated with an elevated risk for autism (
13) and for schizophrenia (
14). It has been estimated that as much as 15% of childhood emotional problems may be attributable to prenatal exposure to maternal psychopathology (
15). At least some of the negative impact of in utero exposure remains even with postnatal treatment of the mother (
16); whether effective prenatal treatment of the mother lowers the risk for her offspring remains unknown.
Antidepressant treatment during pregnancy may be associated with a small elevation in risk of negative birth outcomes, including spontaneous abortion (
17), prematurity (
18), small size for gestational age at birth (
19), persistent pulmonary hypertension of the newborn (
20), and congenital heart defects (
20–
22). Antidepressants cross both the placenta and the blood-brain barrier, yet few studies have examined brain or behavioral outcomes of prenatal maternal antidepressant use. In general, other than a slight delay in motor development (
23), no significant effects of in utero antidepressant exposure on brain and behavior measures have been identified. However, sample sizes for such studies have been small, outcome measures have been nonspecific (e.g., general intelligence scores), and only a limited number of studies have controlled for maternal psychopathology (
18,
23) in order to distinguish the neuropsychiatric effects of antidepressant exposure from the underlying maternal pathology leading to antidepressant use (
20). Attentional deficits are among the most commonly identified deficits associated with in utero exposure to maternal stress; however, many measures of attention, such as cognitive tasks, can be assessed only later in childhood, when the child's rearing by an ill parent may have had its own effects (
24). Hence, in this study, we assessed the development of an evoked potential infant correlate of attentional function, P50 sensory gating, soon after birth in offspring of women with and women without anxiety diagnoses, some of whom were treated with antidepressants.
Repeated stimuli, such as auditory clicks delivered in pairs, engage both excitatory and inhibitory cerebral mechanisms. Diminished response to the second stimulus of the pair occurs because of inhibitory mechanisms activated during the response to the first stimulus. In adults, P50 sensory gating is impaired in a number of psychiatric disorders characterized by attentional dysfunction, including schizophrenia (
25), bipolar disorder (
26), attention deficit hyperactivity disorder (
27), posttraumatic stress disorder (
28–
31), and Parkinson's disease (
32). In families with high rates of P50 sensory gating dysfunction, such as families of individuals with schizophrenia, P50 sensory gating deficits and attentional dysfunction cosegregate (
33). P50 sensory gating is also correlated with attentional functioning within individuals, whether attention is assessed via neurocognitive testing (
34) or based on self-reported ability to selectively attend (
35).
The P50 auditory evoked potential is not fully developed in infants, occurring about 70 msec after the stimulus in infants, rather than the 50 msec generally seen in adult populations. The infant response is also somewhat broader than the adult P50. However, its inhibition to the second of paired stimuli occurs, and, to keep terminology consistent with that used in adult studies, this inhibition is referred to as P50 sensory gating (
36). The infants are recorded in active sleep, a REM-like state in which they spend a majority of their sleeping time. Adults recorded in REM sleep show sensory gating inhibition and deficits similar to those seen in the awake state (
37,
38). P50 sensory gating of 14 children assessed at age 14 weeks was correlated with P50 sensory gating at 47 months (r=0.75, p=0.002), suggesting that sensory gating is stable across early childhood (
39). Elevated ratios (impaired sensory gating) in infancy are also associated with factors that suggest increased vulnerability to attentional deficits, such as increased genetic vulnerability from having a parent with psychosis and in utero exposure to nicotine, an environmental risk factor (
40).
The most common maternal psychiatric illnesses involve anxiety and depression, which are often comorbid. There is some indication that maternal anxiety may be the more significant risk factor for the fetus (
41–
43). The object of this study was to assess the effects of antidepressant exposure on the development of inhibitory brain function in the fetus while accounting for the independent effects of maternal anxiety.
Method
Participants
Initial recruitment screening included the exclusionary criteria of known birth defects, chromosomal abnormality, and infant major neurological disorder. For a total of 313 mother-infant dyads, an infant physiological recording was completed and a maternal structured diagnostic psychiatric instrument was administered. Prenatal exposure to nicotine, nonnicotine substance use, and parental psychotic illness are associated with impaired infant sensory gating (
40,
44); 71 dyads were excluded for presence of at least one of these exposures (56 for tobacco use, 16 for nonnicotine substance use disorder, and 16 for a parental psychotic diagnosis). Of the remaining 242 dyads, 133 (55%) were recruited via a state birth registry, 122 (42%) were recruited from local obstetrics clinics, four (2%) self-referred themselves to the research program, and three (1%) were referred from a local infant care treatment program. Written informed consent was obtained from mothers and participating fathers as monitored by the Colorado Multiple Institutional Review Board. Data on a subset of the mother-infant pairs have previously been reported (
40).
Maternal Diagnosis
Mothers' psychiatric histories were elicited via the Structured Clinical Interview for DSM-IV Axis I Disorders (
45). All interviews were conducted by an experienced psychiatric clinician (a physician or a master's-level social worker), with translation services when necessary. Mothers were considered to have an anxiety disorder if they had any history of chronic or sustained anxiety diagnoses, including agoraphobia, generalized anxiety disorder, obsessive-compulsive disorder, panic disorder, and posttraumatic stress disorder. Mild or intermittent anxiety disorders that could generally be avoided by behavioral changes, such as specific phobia and social phobia, were excluded. Current psychiatric symptoms were defined as a mother having sufficient symptoms to meet criteria for an anxiety or depression diagnosis during her pregnancy or an illness with onset prior to pregnancy with continued symptoms causing impairment during pregnancy. Mothers with previous or chronic illness who were not impaired during pregnancy, regardless of treatment, were considered not to have current anxiety symptoms.
Auditory Sensory Gating
Details of the recording procedures have been reported previously (
36). EEG at site Cz, bipolar electro-oculogram, submental electromyogram, and respiration were continuously recorded while infants slept. Paired clicks were presented through two speakers positioned at either side of the infant's head, approximately 0.5 m from each ear. Auditory clicks were 85 dB (SPL) at the ear. Recording continued for as long as the infant remained asleep.
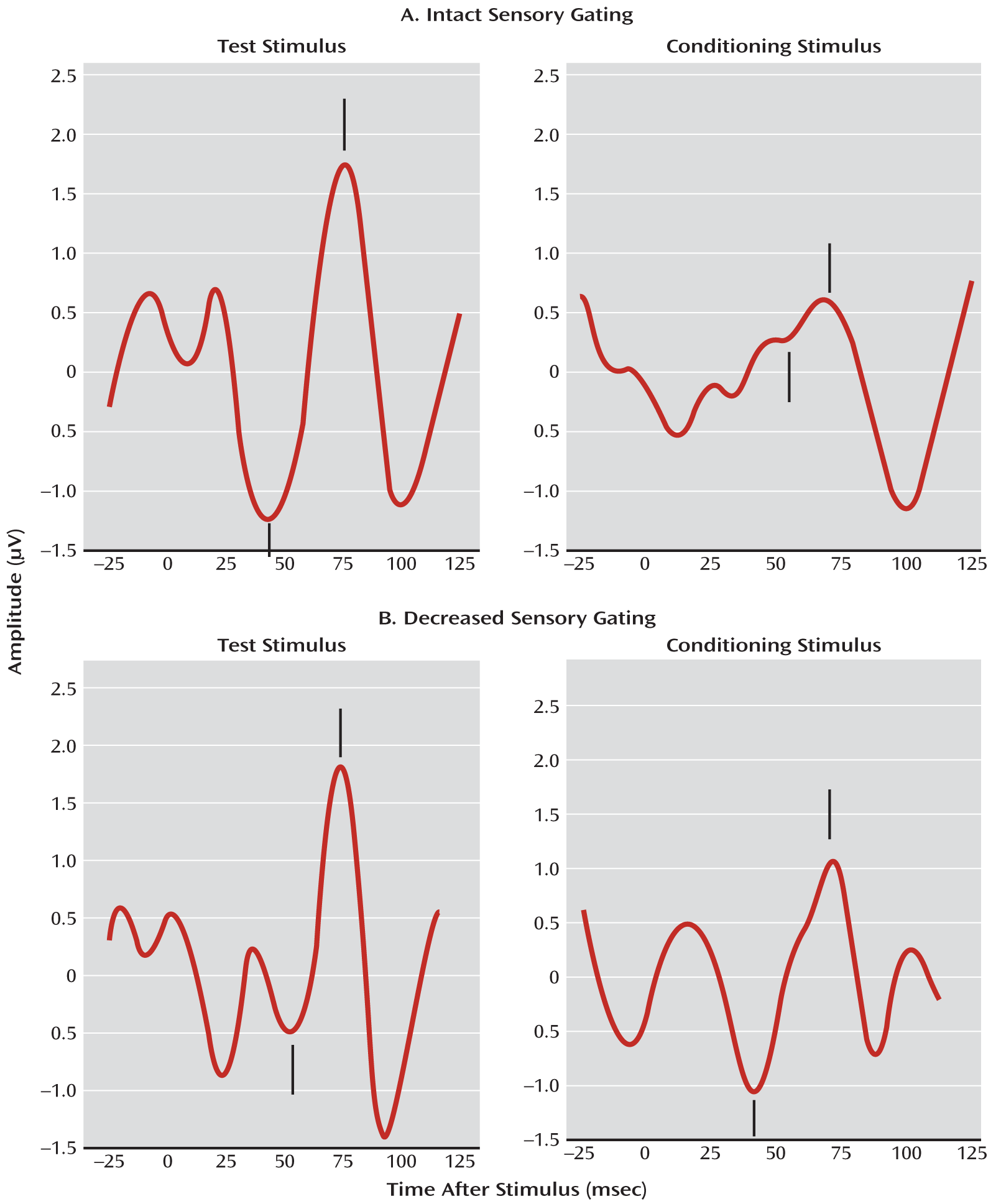
Sleep state was identified offline, and average waveforms were computed from the first 15 minutes of usable active sleep data. The amplitude and latency of the largest positive peak (P1) between 50 and 100 msec following a click and preceded by a negative trough was determined by an investigator who was blind to maternal diagnosis and antidepressant treatment. Sensory gating was measured by dividing the average amplitude of P1 evoked by the second click by the average amplitude of the first click, yielding a P50 sensory gating ratio (
Figure 1). The test/retest reliability of this measure is 0.86 in infants (
46).
Statistical Analysis
Because recruitment was not stratified and assignment to antidepressants not randomized, the two-factor maternal anxiety and maternal antidepressant use analysis had sample sizes ranging from 13 to 169 subjects per cell. The small number of subjects in some cells resulted in relatively low power to test interactions; for an analysis of variance (ANOVA) with an alpha of 0.05, 80% power was achievable only for large interaction effect sizes of 1.18 standard deviations or greater. Insufficient power increases the risk of an interaction effect type II error, which decreases confidence in main effect statistical results. To avoid problems associated with interpretation of main effects in the presence of a potential interaction that cannot be detected because of low power, ANOVA was used to compare the four group cell means, as is common practice when it is known that interaction is present. Each dependent measure—P50 gating ratio and amplitudes of responses to the first and second stimuli—was assessed with separate ANOVAs comparing post hoc differences of least squares group means. The Tukey-Kramer method of multiple comparisons was used where appropriate.
Results
Demographic and Clinical Characteristics
Sixty of the 242 mothers (25%) had a history of anxiety disorder. Mothers with a history of anxiety disorder were more likely than those without to meet diagnostic criteria for current or residual anxiety (63% compared with 0%; χ
2=136.7, p<0.001) and depressive disorders (23% compared with 4%; χ
2=16.7, p<0.001) and to use antidepressants (24% compared with 7%; χ
2=11.9, p=0.001) during pregnancy. On average, mothers with a history of anxiety disorder were also younger, less educated, and of lower socioeconomic status; they were also more likely to have a female infant, but this difference fell short of statistical significance. Infants exposed to maternal anxiety disorder did not significantly differ from those not exposed on race/ethnicity, frequency of living with both biological parents, gestational age at birth, or chronological age at time of physiological recording. Demographic and clinical information for the 242 dyads is summarized in
Table 1.
Twenty-seven (11%) of the women used an antidepressant during pregnancy (see
Table 1). Antidepressant treatment was confined to the first trimester in four women (15% of those utilizing antidepressants), the second trimester in one woman (4%), and the third trimester in six women (22%), and occurred during more than one trimester in 16 women (59%). Nineteen mothers (70% of those exposed during pregnancy) continued taking antidepressants after delivery. Of these, 16 (59% of those exposed prenatally) breast-fed their infants, exposing them to antidepressants postnatally.
Twenty-six of the 27 women (96%) who used antidepressants during pregnancy had a lifetime history of either a mood or an anxiety disorder; however, only 26 of 118 women with a lifetime history of a mood or anxiety disorder (22%) used antidepressants during pregnancy. Of those with a lifetime history of a mood or anxiety disorder, antidepressant use was associated with a nonsignificant elevation in age at delivery (30.9 years compared with 28.6 years; t=1.85, df=116, p=0.067); there were no significant associations with education, socioeconomic status, minority racial or ethnic status, marital status, duration of gestation, or gender of the fetus.
Effects on Infant Sensory Gating
Age at delivery, years of education, and socioeconomic status differed significantly between women with a history of anxiety disorder and those without. All analyses were conducted both with and without these covariates, with no notable difference in results. Analyses without the covariates are reported here.
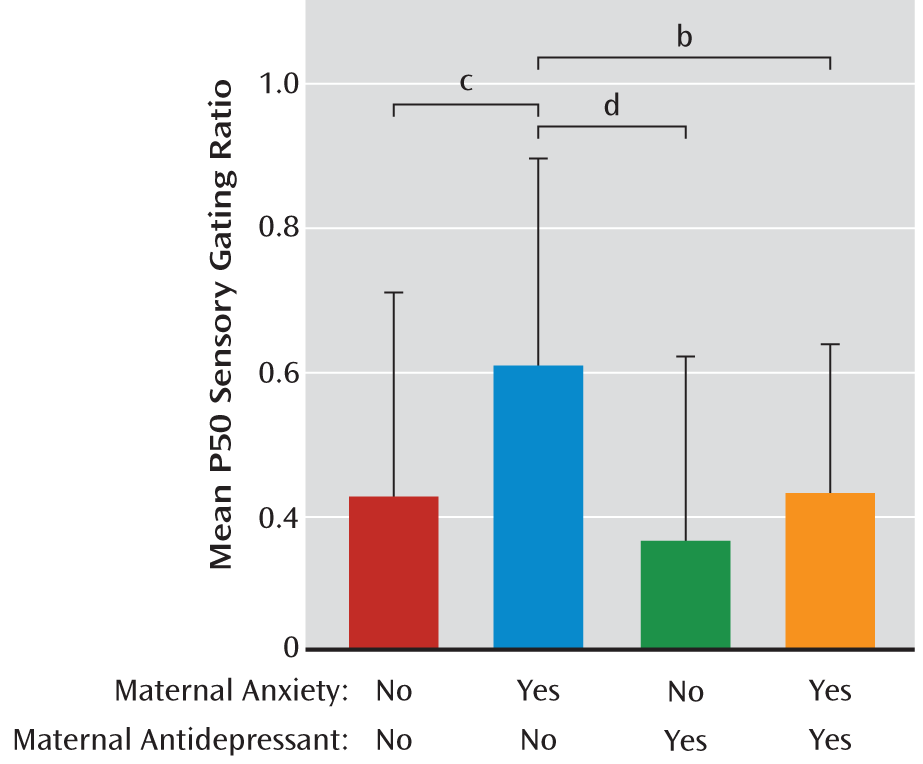
Table 2 summarizes the P50 sensory gating results. For infant P50 sensory gating ratios, there was an overall significant ANOVA among the four groups formed by the presence or absence of a history of maternal anxiety disorder and by the presence or absence of prenatal antidepressant exposure (differences between the four means, F=5.60, df=3, 238, p=0.001). In the absence of antidepressants, maternal anxiety disorder was associated with elevated P50 sensory gating (Tukey-Kramer p<0.001) (
Figure 2). Mean P50 sensory gating ratios were lower in infants of mothers treated with antidepressants, either with or without maternal anxiety disorder, than the mean for infants of untreated mothers with anxiety disorder (Tukey-Kramer p=0.007 for those without maternal anxiety disorder, p=0.041 [uncorrected] for those with maternal anxiety disorder).
The limited sample sizes in the antidepressant-exposed groups restrict additional subgroup analyses. Exploratory analyses did not identify P50 sensory gating differences between infants with pre- and postnatal antidepressant exposure compared to infants with only prenatal exposure or between infants exposed to selective serotonin reuptake inhibitors compared to infants exposed to other antidepressants.
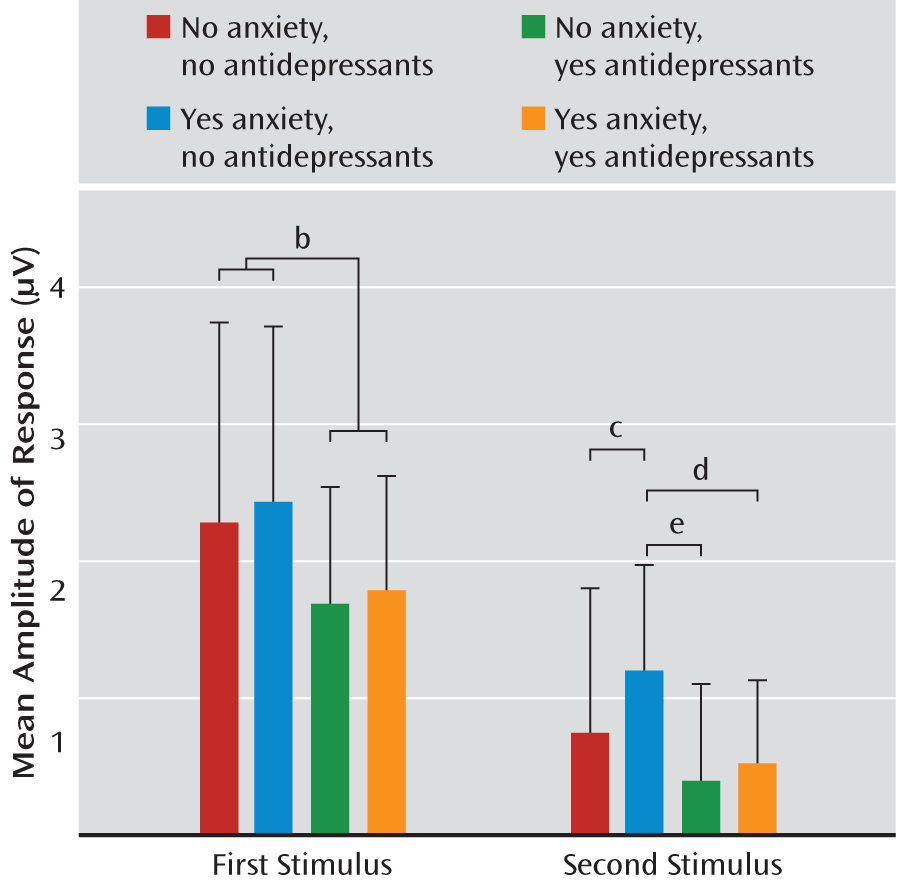
Because P50 sensory gating is calculated as a ratio, it can vary with the numerator (amplitude of response to the second stimulus), the denominator (amplitude of response to the first stimulus), or both. For all four groups of subjects, the amplitude of response to the second stimulus was significantly lower than the amplitude of response to the first stimulus (all p values <0.001), demonstrating the presence of sensory gating in all groups. To further explore the impact of group membership on P50 amplitude, two separate ANOVAs were conducted, one to explore the impact of group on amplitude of response to the first stimulus and the other to explore the impact of group on response to the second stimulus. There was no significant effect of group on P50 amplitude in response to the first stimulus; however, when groups were collapsed across anxiety status, there was a significant effect of antidepressant exposure (t=2.06, df=240, p=0.040) (
Figure 3). An overall significant effect of group was identified for amplitude of response to the second stimulus (F=3.83, df=3, 238, p=0.010) (
Figure 3). Anxiety disorder in the absence of antidepressant use significantly increased the amplitude of infant P50 response to the second stimulus (Tukey-Kramer p=0.005). Antidepressant use in the presence of an anxiety disorder reduced amplitude to levels similar to those found in infants who had no prenatal exposure to either maternal anxiety or antidepressants. Compared to infants of untreated mothers with anxiety, antidepressant use in mothers without anxiety disorder decreased the P1 amplitude in infant response to the second stimulus (Tukey-Kramer p<0.001), and there was indication of the same effect in infants of mothers with anxiety disorder (p=0.026 uncorrected).
Discussion
In the absence of prenatal exposure to antidepressants, infants born to a mother with an anxiety disorder had more impaired P50 sensory gating than infants born to a mother with no identified anxiety disorders. For infants whose mothers had an anxiety disorder, prenatal antidepressant treatment improved sensory gating. Because the effect's significance in mothers with anxiety disorders (p=0.041) was not verified by rigorous correction for multiple testing, the effect of maternal anxiety disorder should be considered mitigated but not fully reversed by antidepressant treatment. Two-thirds of the mothers with an anxiety disorder had continuing symptoms despite treatment, which may explain why treatment was only partially effective. Although tests of equivalence were not conducted, the P50 sensory gating ratios of infants whose mothers took antidepressants (whatever their diagnostic status) were within the range for P50 ratios in infants of untreated mothers with no history of anxiety disorders. Given the limited sample size, we cannot draw any firm conclusions about the relative effect sizes of maternal anxiety versus maternal antidepressant use; however, the effects are in opposite directions and suggest that antidepressant use at least partially mitigates the effect of anxiety. To our knowledge, this is the first report of antidepressant use protecting against adverse effects of a maternal history of anxiety on fetal brain development.
The relationship between prenatal exposure to maternal anxiety disorder and impaired P50 sensory gating is consistent with previous reports that attentional dysfunction is elevated in children prenatally exposed to maternal anxiety (
48,
49). The mechanism by which maternal stress affects fetal brain development is unclear, but there are several possibilities. In older children and adults, anxiety and attentional dysfunction are often comorbid (
50–
52), and shared genetic risk between maternal anxiety and offspring attentional dysfunction is a possibility. Maternal trait anxiety may be associated with alterations in maternal cortisol secretion (
53), and both state and trait anxiety may increase the percentage of maternal cortisol that crosses the placenta (
54). A deleterious direct effect of cortisol on the developing brain is thus a second possibility. Other possibilities include effects of estrogen and other hormones, which affect other measures of sensory gating (
55).
The increased impairment in sensory gating seen in infant offspring of mothers with maternal anxiety disorders is primarily due to an increase in response to the second stimulus, with little or no change in response to the first stimulus. The normal inhibition seen in response to the second stimulus is mediated by a local inhibitory neurocircuit involving GABA-ergic interneurons (
56). Stimulation during early development of α7 nicotinic cholinergic receptors on the interneurons appears critical for normal development of the inhibitory neurocircuit, and elements that interfere with adequate early development, including both genetically mediated decreases in α7 nicotinic receptor density (
57,
58) and decreases in the prenatal ligand choline (
59), permanently impair inhibition to the second stimulus, increasing the resulting P50 sensory gating ratio. Stress leads to decreased serum choline levels (
60–
63), primarily as a result of corticosteroid-mediated increased sequestration of choline in the liver (
64). Maternal anxiety disorders may result in a cortisol-mediated drop in maternal and fetal serum choline levels, resulting in decreased fetal brain α7 nicotinic receptor stimulation, diminished development of inhibitory neurocircuits, and a resultant impairment in the ability to gate the response to repetitive auditory stimuli.
Although our analysis did not survive rigorous adjustment for multiple testing, our results suggest that antidepressant exposure may be associated with improved P50 sensory gating. In adults, P50 sensory gating is impaired in those with a history of mood disorder but is not correlated with current mood state (
65,
66). Antidepressant use in adults has been associated, in some but not all studies, with normalization of sensory gating even when mood symptoms were not fully treated (
66,
67). One possibility is that improvement in infant P50 sensory gating related to antidepressant exposure is an acute effect of current antidepressant exposure; however, infants with pre- and postnatal antidepressant exposure did not differ in P50 sensory gating ratios from those with prenatal exposure only. Thus, postnatal exposure effects seem unlikely.
The mechanisms by which antidepressants could exert an effect on the fetal brain are unknown, but they could include a reduction in the duration or severity of the mother's symptoms or direct protective effects on the fetal brain. Antidepressant exposure was associated with reductions in evoked P50 response amplitude in response to both the first and second stimuli. As discussed above, response to the second stimulus is associated with α7 nicotinic receptor function; response to the first stimulus appears to be more closely related to α4β2 nicotinic receptor activity (
68), at least in adult animal models. A pharmacological feature common to most antidepressants, including selective serotonin reuptake inhibitors, tricyclics, bupropion, and venlafaxine, is noncompetitive inhibition of a broad range of nicotinic receptors, including α7 and α4β2 (
69–
76). The fact that both α7 and α4β2 nicotinic receptors play a role in local inhibitory neurocircuit formation and function, and that antidepressants are noncompetitive inhibitors of both receptor types, suggests one avenue for additional research.
Our study had several limitations. Only 27 participants (11% of the sample) took antidepressants during pregnancy. When combined with an anxiety history classification, some subgroups were as small as 13 participants, limiting statistical power. In addition, while antidepressant use was almost completely limited to women with a history of either a mood or an anxiety disorder, only a minority of women with such a history used antidepressants, and antidepressant use was not randomized. Among women who had a history of mood and anxiety disorders, there was no difference on several demographic variables between those who did and those who did not take antidepressants; however, there are likely other factors that influence the choices individuals make on pharmacological treatment that were not identified or controlled for in this study. Additional efforts to replicate and extend the findings of this study should account for other potential confounding factors to establish the risk and benefit to the fetus of maternal antidepressant treatment.