Anorexia nervosa and bulimia nervosa are disorders of unknown etiology that tend to affect young women. They are characterized by extreme eating behavior and distorted body image and have high rates of chronicity, morbidity, and mortality. Several lines of evidence implicate genetically mediated neurobiological factors as contributing to the development of anorexia nervosa and bulimia nervosa (
1,
2). However, a lack of understanding of the pathophysiology of these illnesses has hindered development of effective treatments.
How are individuals with anorexia nervosa able to consume a few hundred calories per day and maintain an extremely low weight for many years, when most people struggle to lose a few pounds? And why do individuals with bulimia nervosa, who are often of normal weight, binge on thousands of calories per day? Although both are categorized as eating disorders, it is unknown whether individuals with anorexia nervosa and bulimia nervosa have a primary disturbance of appetite regulation or whether pathological feeding behavior is secondary to other phenomena, such as an obsessional preoccupation with body image. Recent studies of obesity suggest that corticolimbic neural processes, which encode the rewarding, emotional, and cognitive aspects of food ingestion, can drive overconsumption of food, even in the presence of satiety and replete energy stores (
3,
4).
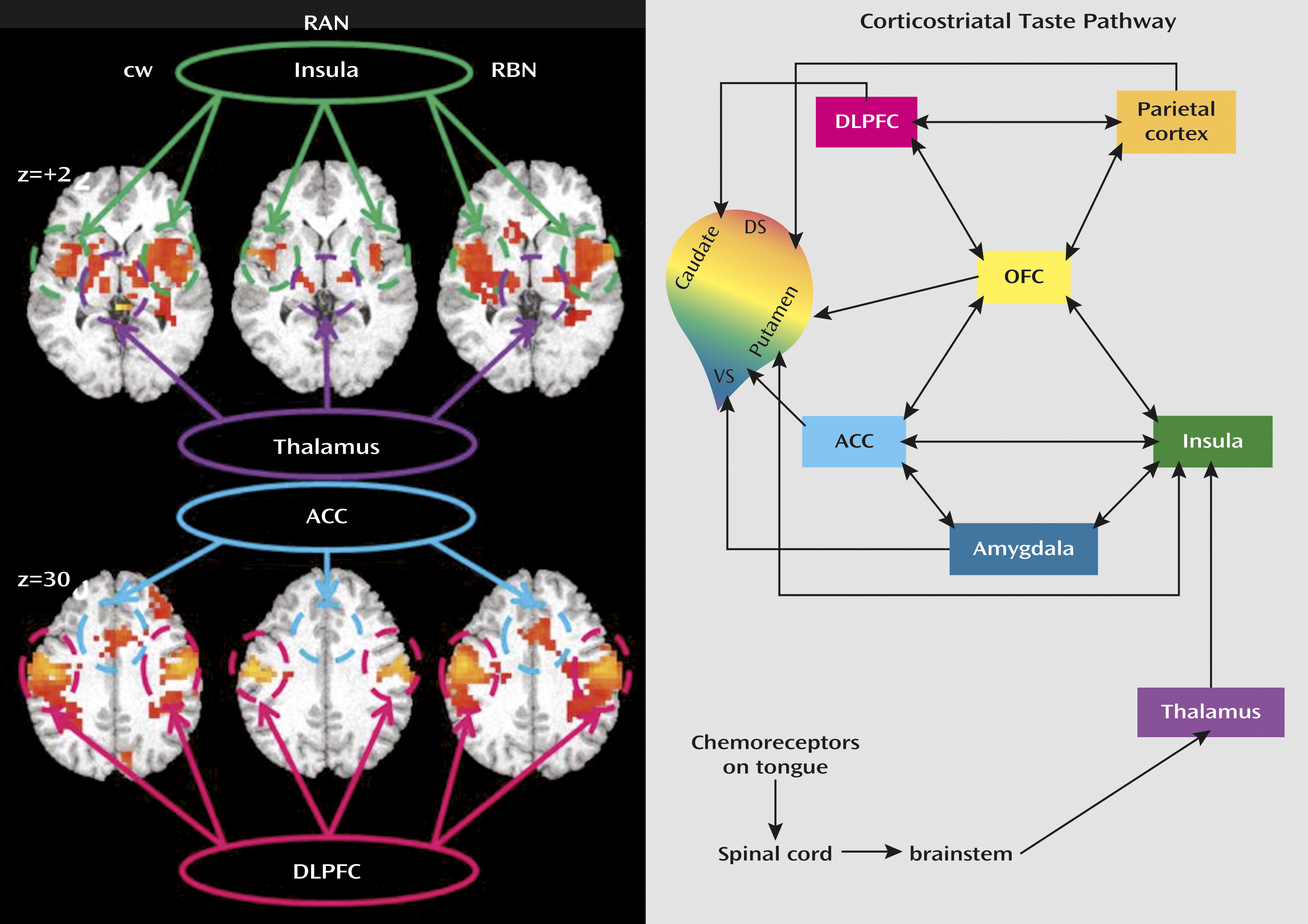
To determine whether corticolimbic circuits are involved in appetite regulation in eating disorders, we interrogated the neural circuitry of gustatory processing, which integrates the sensory, hedonic, and motivational aspects of feeding, after a modest meal (
Figure 1) (
1,
4,
5). As Small has noted (
6), this circuit can be assessed by using a sweet taste stimulus. Sweet taste perception is peripherally recognized by the tongue’s sweet taste receptors, from which signals are transmitted through the brainstem and thalamus to the primary gustatory cortex, which comprises the frontal operculum and anterior insula. The anterior insula and associated gustatory cortex respond to the taste and physical properties of food and may also respond to its rewarding value. The subgenual anterior cingulate cortex is linked to hypothalamic and brainstem pathways that mediate autonomic and visceral control. The orbitofrontal cortex is associated with flexible incentive responses to changing stimuli. These regions innervate a broad region of the rostral ventral-central striatum, where behavioral repertoires are computed based on these inputs.
When individuals with anorexia or bulimia nervosa are in an ill and symptomatic state, they have disturbances of most physiological systems. This confounds the determination of whether abnormal ill state findings are a cause or a consequence of starvation. In order to avoid confounding effects, we studied individuals recovered from restricting-type anorexia nervosa or bulimia nervosa and matched healthy comparison women. Approximately 50% of individuals who have anorexia and bulimia nervosa recover in the sense that their weight and nutritional status normalize (
7), although persistent mild to moderate dysphoric mood, obsessional thoughts, and body image concerns are common (
8). Because these symptoms are present in childhood, before the onset of the eating disorder, they may reflect traits that contribute to a vulnerability to develop anorexia or bulimia nervosa.
We used functional MRI (fMRI) and sweet taste administration to interrogate top-down sensory-interoceptive-reward processes. The whole-brain functional analysis was constrained to the anatomical regions of interest based on the Talairach atlas. We sought to replicate an earlier finding from our group (
9) showing that women recovered from anorexia nervosa have reduced insula and striatal response to tastes of sucrose, which suggests diminished response of the sensory reward circuitry responsible for consummatory drive. Because individuals with bulimia nervosa have a drive to overconsume, we hypothesized that they would have an exaggerated response of sensory-reward circuitry.
It is possible that the brain differentially processes sweetness compared with the caloric content of sucrose. For example, the hypothalamus responds to the caloric content of sugar (
10). Thus, the noncaloric sweet solution sucralose was chosen as a contrast condition because it is similar to sugar in taste, molecular makeup, and recognition by tongue sweet receptors (
11) but lacks its caloric properties (
12). Disentangling processes related to sweetness and those related to caloric content may help us understand why individuals with anorexia or bulimia nervosa have strong emotional responses to high-calorie foods.
Method
Participants
We studied 14 women recovered from anorexia nervosa, 14 women recovered from bulimia nervosa, and 14 age- and weight-matched healthy comparison women (
Table 1). Trained clinicians administered the Structured Clinical Interview for DSM-IV Axis I Disorders (
13) to assess inclusion and exclusion criteria and to characterize lifetime history of comorbid psychiatric disorders (for comorbidities, see the
data supplement that accompanies the online edition of this article). Participants completed the Beck Depression Inventory (
14) to assess depression, the Temperament and Character Inventory (
15) to assess harm avoidance, and the State-Trait Anxiety Inventory, form Y (
16), to assess state and trait anxiety. Women recovered from anorexia nervosa had lost weight purely by restricting their diet and had no history of binge eating or purging. Women recovered from bulimia nervosa had a history of past binge eating and purging behaviors but had never been emaciated and had maintained an average body weight above 85% of ideal body weight. None of the participants had a previous history of both anorexia nervosa and bulimia nervosa. Recovered participants were required to have had no restrictive eating or other pathological eating-related behaviors in the preceding 12 months; a stable weight between 90% and 120% of ideal body weight for at least 12 months; regular menstrual cycles for the preceding 12 months; and normal concentrations of plasma β-hydroxybutyric acid glucose and insulin during the evaluation phase, as previously described (
8). Comparison women had no history of an eating disorder or any other psychiatric disorder, no history of serious medical or neurological illness, and no first-degree relatives with an eating disorder, and they had been within normal weight range since menarche. All participants had normal menses and were studied during the early follicular phase of the menstrual cycle. Participants took no medications within 30 days before the study. After receiving a complete description of the study, all participants gave written informed consent. The University of California San Diego institutional review board approved the study.
Imaging Procedures
Participants were instructed to fast overnight and to arrive at the fMRI facility between 7:00 and 8:00 a.m. They received a standardized breakfast of 604 calories before scanning to control for satiety state.
Taste Solution Delivery
Sucrose and sucralose solutions were delivered with a programmable syringe pump in 1 mL/second stimulations. Participants received 1 mL of either sugar or sucralose every 20 seconds for a total of 120 samples. Sweet tastes were matched for intensity and delivered in pseudorandomized order. (Additional methods, as well as other relevant issues, are described in the online
data supplement.)
fMRI Acquisition
Scanning was performed on a 3-T GE Magnet, with a three-plane scout scan (16 seconds), a sagitally acquired spoiled gradient recalled sequence (T1-weighted, 172 slices, thickness=1 mm, TI=450 ms, TR=8 ms, TE=4 ms, flip angle=12°, FOV=250×250 mm, 192×256 matrix interpolated to 256×256), and T2*-weighted echo planar imaging scans to measure blood-oxygen-level-dependent (BOLD) functional activity during taste stimulation (3.43×3.43×2.6 mm voxels, TR=2 seconds, TE=30 ms, flip angle=90°, 32 axial slices, thickness=2.6 mm, gap=1.4 mm).
fMRI Preprocessing
Images were processed with the AFNI (Analysis of Functional Neuroimages) software package (afni.nimh.nih.gov/afni/). To minimize motion artifact, echo planar images were realigned to the 100th acquired scan. Additionally, data were time-corrected for slice acquisition order, and spikes in the hemodynamic time course were removed and replaced with an interpolated value from adjacent time points using 3dDespike. A multiple regression model was used whereby regressors derived from the experimental paradigm were convolved with a prototypical hemodynamic response function (AFNI command “waver”), including five nuisance regressors: three movement regressors to account for residual motion (roll, pitch, and yaw), and regressors for baseline and linear trends to account for signal drifts. To account for individual anatomical variations, a Gaussian filter with full width at half maximum 6.0 mm was applied to the voxel-wise percent signal change data. All functional data were normalized to Talairach coordinates.
fMRI Analysis
Single-sample t tests were performed on the main effects of sucrose and sucralose, and statistical maps were thresholded at a p value of 0.005. Both whole-brain and region-of-interest analyses were performed. The whole-brain analysis was thresholded at 2,048 mm
3 (32 voxels) and masked to a priori regions of interest implicated in taste and reward processing (see Table S1 in the online
data supplement). A threshold adjustment method based on Monte Carlo simulations was used to guard against identifying false positive areas of activation (AFNI program AlphaSim). Voxel-wise percent signal change data were entered into a group-by-condition (sucrose/sucralose) analysis of variance (ANOVA) test thresholded at a p value of 0.05. Percent signal change data from statistically derived regions of interest were used to assess correlation and regression analyses with behavioral data.
Discussion
To our knowledge, this is the first fMRI study to compare sweet taste response between women recovered from anorexia nervosa and bulimia nervosa and comparison women. Within corticolimbic circuits involved in appetite regulation (
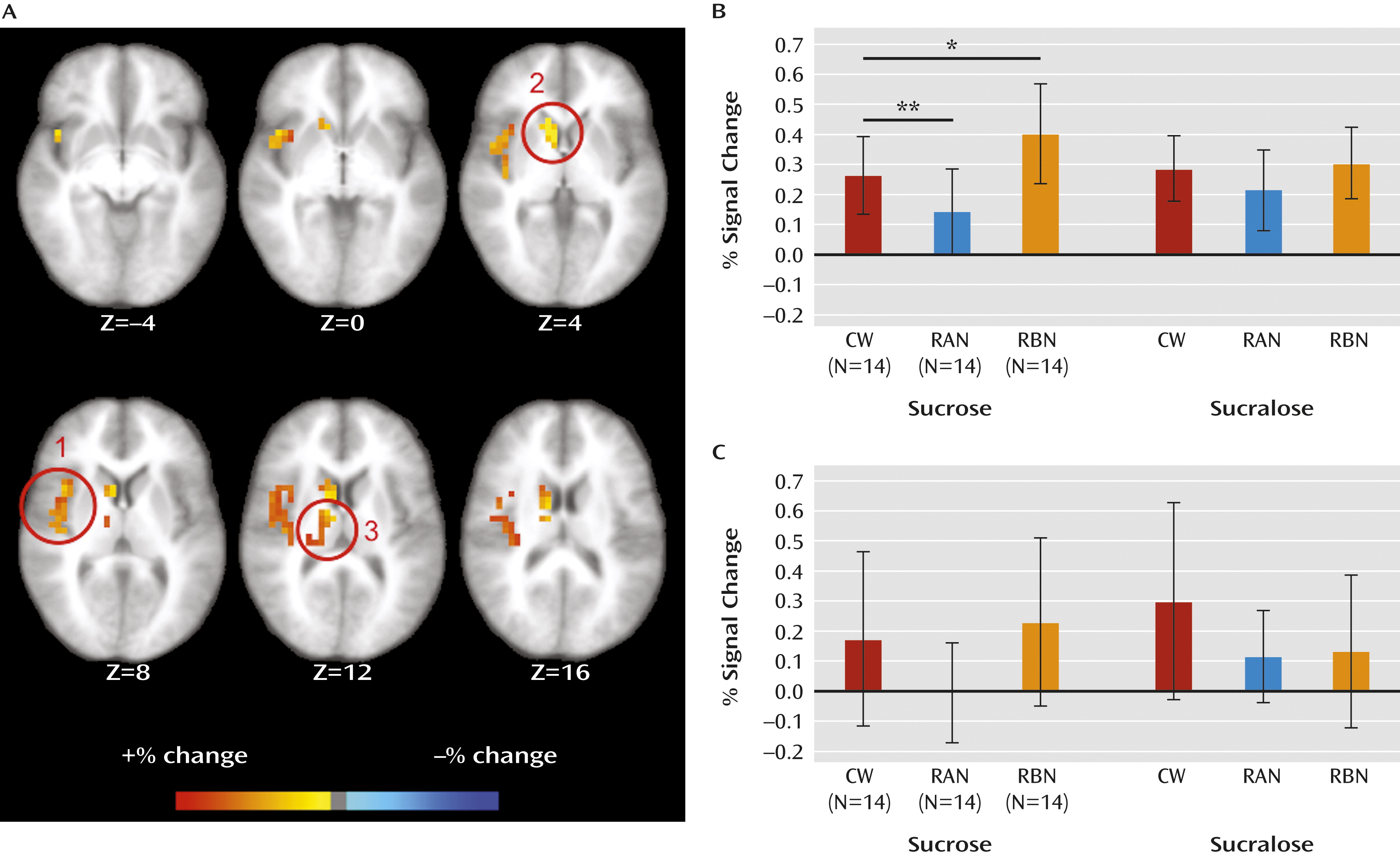
Figure 1), we found that right anterior insula response to sucrose was diminished in women recovered from anorexia nervosa and exaggerated in women recovered from bulimia nervosa relative to comparison women.
Other studies investigating response to tastes of foods have shown abnormal insula response in anorexia nervosa. We replicated a previous study from our group that found that women recovered from anorexia nervosa had diminished hemodynamic response in the anterior insula to tastes of sucrose or water (
9). Vocks et al. (
18) compared hunger and satiety states while drinking chocolate milk and showed that, in the hungry state, patients with anorexia nervosa exhibited less insula activation than healthy comparison subjects.
The anterior insula is well established as the primary taste cortex (see the online
data supplement), which integrates the sensation of taste with multiple bodily sensations to generate an “internal milieu” or the “interoceptive state” (
6,
19). The anterior insula, as part of the limbic sensory cortex (
20), is involved in representations of the hedonic state of the individual. Thus, altered neural signaling in the anterior insula could suggest a dysregulation of hedonic taste processing in individuals with eating disorders. Previous investigations have shown normal perception of sweet taste in patients with eating disorders (
21). Instead, consistent with previous studies (
21) (see the
data supplement), our results of altered sweet taste preference support the hypothesis of altered neural representation of hedonic valuation in eating disorders.
The connectivity of the anterior insula with other brain areas that are important for reward-related processing implies that the emotional value of interoceptive cues, such as taste or feelings of hunger or fullness, are computed in the anterior insula (
20). This brain area has been implicated in a contextualized representation of the “feeling” self in time and with respect to maintenance of a homeostatic state. For example, a current state, such as food deprivation, is compared with a previous state of homeostasis, and this information is then integrated in the formation of emotions (
22,
23). Sweet tastes, such as those delivered in our study, are processed against this “interoceptive-hedonic” backdrop.
Brain imaging studies giving tastes of sugar to healthy individuals in food-deprived compared with satiated states have consistently shown that receipt of sucrose in the food deprivation state results in relatively higher activation in the insula and orbitofrontal cortex, potentially reflecting perceived change in interoceptive state (
24) (see the
data supplement). In the present study, we fed subjects a modest meal before fMRI scanning and thus did not test extremes of feeding or food deprivation. Still, these data raise clinically relevant questions with regard to how symptoms in eating disorders may be related to erroneous interoceptive feedback. The relatively lower activation of insula signal in individuals recovered from anorexia nervosa suggests a signal consistent with relatively high satiation. Attenuated anterior insula activation in anorexia nervosa could therefore reflect a hunger signal that is attenuated compared with the fed state. In a clinically ill population, the attenuated signal associated with sweet taste may not provide a sufficient learning signal to change behavior (e.g., eating more in the state of food deprivation), resulting in a rigid behavioral phenotype.
From another perspective, individuals with anorexia nervosa may simply fail to accurately recognize hunger because of altered homeostatic interoceptive signals. There might be a discrepancy between their perceived internal body state (full, bloated) and their actual internal body state (calorie-deficient) causing them to avoid food when internal cues should in fact be driving them to eat. In contrast, based on data from healthy subjects (
24), the relatively higher insula activation in our participants who had recovered from bulimia nervosa may be consistent with the opposite: exaggerated hedonic response to sweet taste together with an interoceptive status of relative hunger. Increased anterior insula activation in bulimia nervosa could represent an exaggerated interoceptive perception of hunger/deprivation signal, which may mutually amplify the reward and homeostatic systems, leading to excessive episodic food intake. While not investigated in this study, it is also possible that other eating disorder symptoms, such as body image distortion, alexithymia, and lack of insight and motivation to change, could be part of a more generalized disturbance related to interoceptive processing (
9).
Dorsal Caudate Response
Our study demonstrated a trend toward reduced dorsal caudate response to tastes of sucrose and sucralose in women recovered from anorexia nervosa. This parallels the insula findings and is in accordance with previous work showing that women recovered from anorexia had diminished hemodynamic response to tastes of sucrose and water bilaterally in the dorsal caudate, dorsal putamen, and ventral putamen (
9). Another study in women recovered from bulimia nervosa (
25) showed an exaggerated anterior ventral striatum response for a cream/water contrast compared with women recovered from anorexia nervosa and healthy comparison women. The striatum receives direct inputs from the insula (
26,
27), and this path is thought to mediate eating behavior, which may have a direct impact on the types of palatable foods avoided or overconsumed in eating disorders (
28). These striatal findings, in conjunction with insula alterations, raise the possibility that there may be a disturbance in the mechanisms translating interoceptive signals into enhanced or diminished motivated eating.
Sweetness Versus Energy Content of Sucrose
Women recovered from anorexia or bulimia nervosa also showed differences in response to sucrose and sucralose in the right anterior insula and right dorsal caudate. No group showed hypothalamic differences. In the right anterior insula, women recovered from anorexia nervosa showed greater response to sucrose than to sucralose, while women recovered from bulimia nervosa showed greater response to sucralose than to sucrose (data not shown). This suggests that sucralose as a contrast solution distinguished between processing of caloric and noncaloric sweet tastes. In contrast, for comparison women, sucrose was similar to sucralose in the anterior insula, and sucralose showed greater activation in the caudate. A previous analysis of data from healthy subjects (
29) supports the speculation (
30) that sucrose, through effects on insula signaling, may result in changes of dopamine transmission that could modify the relative association of the energy value of sucrose to its motivational value. These findings raise the possibility that individuals with anorexia or bulimia nervosa have altered balance or sensitivity regarding mechanisms that signal the caloric content of foods as opposed to gustatory pathways that code the sweetness of foods.
Limitations
The study of eating disorders frequently raises questions regarding cause and consequence: Do neurobiological disturbances cause pathological eating behaviors, or are neurobiological disturbances secondary to abnormal nutrition? Our literature review reveals some consistency of results in ill and recovered individuals with eating disorders, suggesting that these findings could be traits, but there is sparse evidence from direct comparisons using the same design, and no longitudinal studies have been reported. Even if persistent psychophysiological disturbances in recovered eating disorders are “scars,” they are still likely to help us understand the processes contributing to these disorders. These ideas could be tested in children at risk for eating disorders, to see if these neural signatures are predictive of illness development. It should be noted that there are discrepant insula findings in other gustatory studies (
31–
34) that might be related to anticipatory responses (
35) (see the online
data supplement). Our study task did not include an explicit expectation phase, which may contribute to some discrepant results across studies. Other fMRI studies of appetite in eating disorders have employed designs that used pictures of food or food words (
36,
37). Pictures may elicit different brain responses, so this literature is not examined here. Artificial sweeteners are not identical to sugars in terms of how they activate tongue sweet receptors (
38) (see the
data supplement). It is possible that a mismatch in sweetness, rather than added caloric content, contributed to the observed differences between sucrose and sucralose. The use of atlas-based region-of-interest analysis may limit the capacity to detect signal in regions where recovered individuals show differences in brain structure (
39). Although the Gaussian filter that was applied helps correct for some level of individual differences in brain structure, an attenuated or absent effect may represent a false negative resulting from group deviations from the standardized atlas. While the sample size of each cohort was relatively modest, the findings were robust. Compared with other behavioral disorders, eating disorders are characterized by homogeneous symptoms, so that smaller samples may be adequate to show group differences.
Treatment Implications
Aberrant function of the right anterior insula, which integrates gustatory stimuli and interoceptive/hedonic signals, may contribute to a failure of higher-order appetitive processes to reach homeostasis and thus lead to pathological eating behaviors. Attenuated anterior insula response in anorexia nervosa and exaggerated insula activity in bulimia nervosa raise the possibility that these disorders comprise, respectively, an overly rigid or highly unstable neural representation of internal feeling states at the junction of feeding and, possibly, emotive decision making. Identifying abnormal neural substrates in these individuals helps to reformulate the basic pathology of eating disorders and offers targets for novel approaches to treatments. It may be possible to modulate the experience by enhancing insula reactivity when individuals with anorexia nervosa engage in eating behavior, or dampening exaggerated or possibly unstable responses to food in individuals with bulimia nervosa. One approach is the use of techniques that might “train” the insular cortex. Studies have shown that healthy subjects can use real-time fMRI to control right anterior insula activity (
40). Biofeedback might also be useful since there is evidence that it helps individuals with anxiety disorders observe inaccuracies in perceiving physiological activity or strengthen perception when actual somatic changes occur (
41).
It may also be possible to modify existing therapies so they can better target eating disorder psychopathology. For example, it may be possible to behaviorally modulate the insula by increasing the influence of top-down modulation—that is, inhibit the urge to eat in bulimic individuals by employing cognitive training. Alternatively, studies suggest that mindfulness training alters cortical representations of interoceptive attention (
42,
43), or a dialectical behavioral therapy approach may be used to promote development of more effective strategies for recognizing, predicting, and constructively managing tendencies to have rigid or unstable responses to stimuli.
From another perspective, if individuals with anorexia nervosa have an overly active satiety signal in response to palatable foods, it may be worthwhile to try strategies such as avoiding highly palatable foods in favor of bland, dilute, or even slightly aversive foods, and perhaps recommending multiple small meals of equivalent daily calories, in order to avoid overstimulation and early satiety. Finally, pharmacological modulation of insular reactivity might increase sensitivity to food in patients with anorexia nervosa or attenuate hyperresponsivity in those with bulimia nervosa. For example, recent studies in healthy subjects (
44) show that olanzapine enhances reward response to food in brain reward circuitry and decreases inhibition to food consumption in regions thought to inhibit feeding behavior. In summary, an understanding of the basic pathophysiology of anorexia and bulimia nervosa provides rationales and heuristics to develop improved treatments for these chronic and deadly disorders.