Bipolar disorder is a chronic, highly debilitating disease. Only 24% of bipolar patients achieve functional recovery 1 year after a manic episode (
1), a rate that rises to only 43% after 2 years (
2). The level of psychosocial functioning in bipolar patients is correlated with a number of neuropsychological deficits in executive function, attention, and memory (
3,
4). These cognitive deficits are present in manic and depressive episodes and even persist into remission (
5,
6). It has been hypothesized that the cognitive deficits in bipolar disorder arise, at least in part, from a general hyperreactivity of limbic brain structures and emotional hyperreactivity that interferes with neocortical activity necessary for successful task performance (
7,
8). Indeed, heightened amygdala activation at rest and during nonemotional and emotion-related tasks has been seen in symptomatic (
9,
10) and euthymic bipolar patients (
11–
13).
A few studies have investigated cognitive task performance in the presence of emotional distractors. Two studies using the emotional Stroop task found abnormally increased frontal and limbic activity in bipolar patients during color naming of emotional compared with neutral words (
11,
14). However, emotion did not affect color-naming response latencies, both in patients and in comparison subjects, suggesting that task processing was not directly influenced. In a previous study (
15), we applied an emotional go/no-go task, which yielded behavioral distraction effects in the emotional compared with the neutral condition, but no behavioral alterations in bipolar disorder, although activation was increased in frontal and limbic regions. Similar activation increases without behavioral effects were found in working memory tasks with sad mood induction (
16) and face distractor stimuli (
17). Critically, these studies could not clarify whether the observed hyperactivations reflect altered processing of the cognitive tasks (e.g., as a compensatory effect) or simply aberrant emotional processing. Addressing this issue would require independent definition of the relevant task network before studying the influence of emotion on this network.
Furthermore, it is unknown whether the potentially increased emotional distractibility is a consequence of the disorder or whether it represents a vulnerability marker. Clinically, this differentiation is critical for refining etiological models of bipolar disorder and designing either targeted therapy or preventive interventions.
In the present study, we aimed to investigate emotional distractibility in patients with bipolar disorder using a two-step functional MRI (fMRI) procedure. The first experiment allowed for the localization of the neural network relevant to a cognitive task, here mental arithmetic. To elucidate the influence of emotion on this network, the second experiment presented arithmetic tasks on top of emotional and neutral distractor images. Using this procedure, we previously found that in healthy subjects, emotional distraction leads to slower performance and increased activation in the task-related neural network, particularly in the parietal cortex (
18). The crucial role of this region in arithmetic processing has been repeatedly demonstrated (
19,
20). Our approach to testing whether potential deficits represent a vulnerability marker was to investigate remitted patients with bipolar I disorder and two groups of participants at heightened risk for developing bipolar disorder (i.e., unaffected first-degree relatives of bipolar I patients and healthy participants with hypomanic personality [defined using the Hypomanic Personality Scale (
21)]), who have been demonstrated to have an increased risk of developing bipolar disorder over time (
22). We compared each of these experimental groups with separate matched comparison groups and additionally compared the experimental groups directly with demographic variables as covariates.
If cognitive processing deficits in bipolar disorder are associated with increased emotional distractibility, we would expect an exacerbated behavioral slowing effect and a more pronounced task-related activation increase, particularly in the parietal cortex, which should also be present in the high-risk groups if these deficits represent a vulnerability marker of bipolar disorder.
Method
Participants
All participants underwent the Structured Clinical Interview for DSM-IV (
23–
26), as well as screening for exclusion criteria (neurological disorders, head trauma with loss of consciousness, metal implants, tattoos, substance abuse or dependence, and age <18 or >65). Interviews and observer rating scales for mania (Young Mania Rating Scale [
27]) and depression (Hamilton Depression Rating Scale [HAM-D] [
28]) were conducted by clinical psychologists, and participants completed the Beck Depression Inventory (BDI [
29,
30]). The study was approved by the research ethics committee of the Heidelberg University Faculty of Medicine in Mannheim, Germany. All participants provided written informed consent before entering the study.
Sample 1: bipolar patients and healthy comparison subjects.
Twenty-two euthymic bipolar patients and 22 gender-, age-, and education-matched healthy comparison subjects with no history of or current mental disorders participated in the study. Patients were recruited at the Central Institute of Mental Health, Mannheim, Germany, and through local psychiatrists, psychotherapists, and patient support groups. None of the patients currently met criteria for any DSM-IV disorder other than bipolar I disorder. A life chart assessing variables related to illness course was completed for all patients (
Table 1). Euthymia was defined as a HAM-D score <5 and a Young Mania Rating Scale score <7 (
31). We asked patients about their current medication and verified its stability during the past 6 months. To analyze the psychotropic medication effect, we calculated the total medication load according to a published algorithm (
32) reflecting dosage and a variety of different medications (
33). The composite measure was generated by summing all individual medication codes for each medication category (mean=2.32, SD=2.08). We then checked for correlations of this index with the effects of interest in bipolar patients.
Sample 2: relatives of bipolar patients and healthy comparison subjects.
Seventeen unaffected relatives of bipolar I patients and 17 gender-, age-, and education-matched healthy volunteers participated (
Table 1). None fulfilled criteria for any lifetime or current mental disorders, and none were taking any psychotropic medications. The relatives were not related to the bipolar patients tested in this study. Five relatives were siblings, four were children, and eight were parents of bipolar patients. Twelve relatives were from simplex families (one case in the family), and five were from multiplex families (at least two cases in the family).
Sample 3: individuals with hypomanic personality and healthy comparison subjects.
In previous studies (
22,
34), individuals with hypomanic personality were selected based on the Hypomanic Personality Scale, on which scoring in the upper 10% was defined as hypomanic personality (resulting in scores ≥31). Through online questionnaires and in lectures, 1,567 individuals were screened. Twenty-four individuals with hypomanic personality were included, as well as 24 gender-, age-, and education-matched healthy comparison subjects who did not score 0.5 standard deviations above the mean of the distribution (
Table 1). For analyses, two participants with hypomanic personality were excluded because of fMRI motion artifacts. None of the participants in this sample fulfilled criteria for mental disorders or reported a positive family history of affective or psychotic disorders, nor were they taking any psychotropic medications. In general, participants with hypomanic personality reported increased caffeine and alcohol consumption; however, none of the study participants consumed alcohol or caffeine beginning the evening of the day before scanning.
Experimental Paradigm
The details of the experimental paradigm have been described elsewhere (
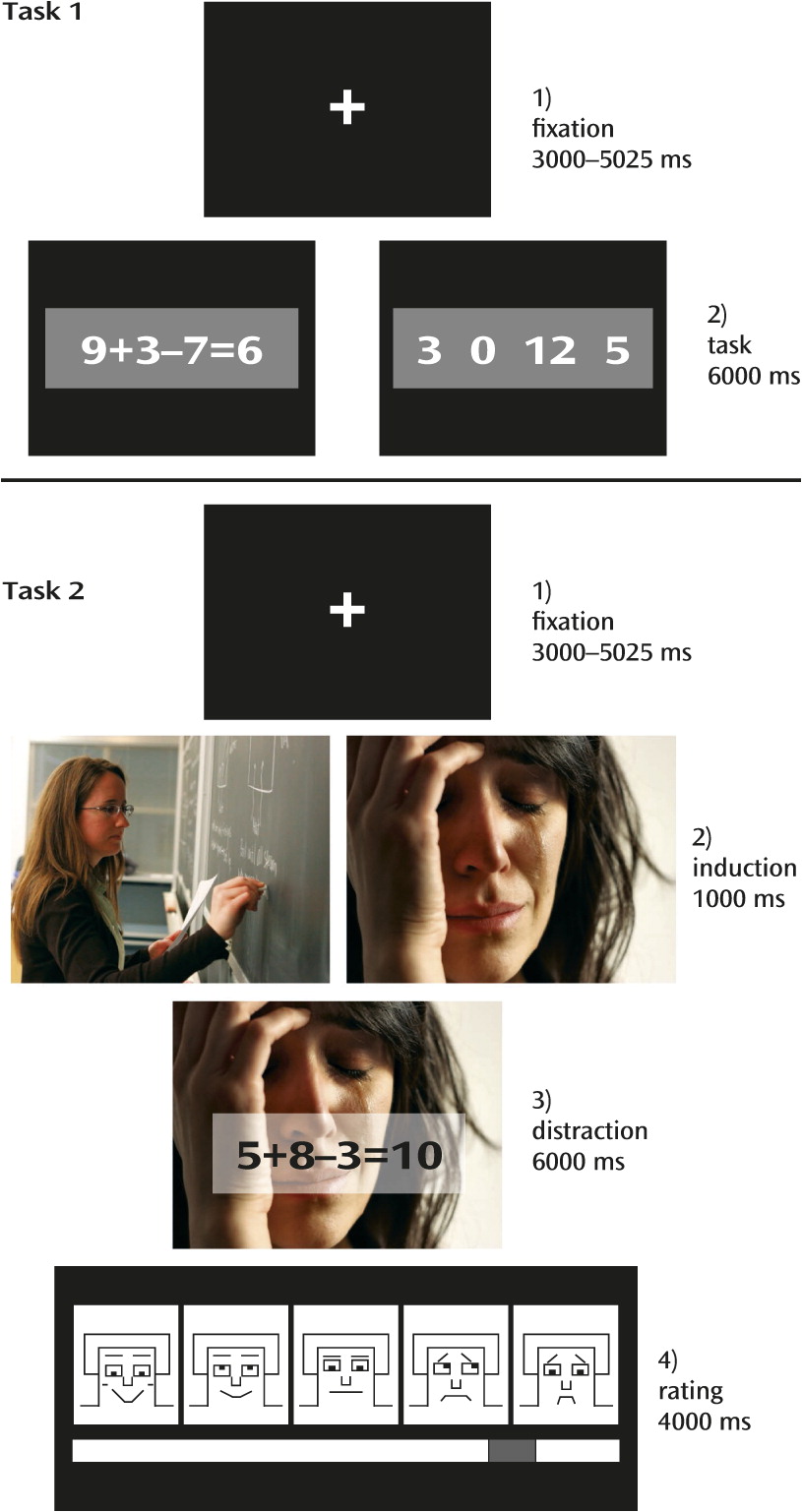
18). Briefly, two experimental tasks were presented (
Figure 1). Task 1 was a localizer to identify brain activation patterns specific to mental arithmetic operations. In this task, 20 arithmetic problems and 20 number rows were presented for 6,000 ms each, and participants decided whether the presented solution for the equation was correct or whether a “0” was part of the number row.
Task 2 aimed to test the influence of emotional distraction on task processing and presented arithmetic problems equivalent to but different from the problems presented in the localizer task (
35). The arithmetic problems were superimposed on 32 highly arousing emotional images (16 negative, 16 positive) and 16 low-arousal neutral images (taken from the International Affective Picture System [
36]). Each trial started with 1) a fixation cross presented with a jitter of 3,000–5,025 ms, followed by 2) an emotion induction phase (1,000 ms), 3) the distraction (i.e., the presentation of an arithmetic problem through a transparent overlay on the images; 6,000 ms), and 4) a rating phase (4,000 ms), which was not relevant to this study. Task 2 included 48 mental arithmetic trials. Participants received six training trials before the experiment to familiarize themselves with the procedure.
MRI Data Acquisition
MRI data were collected on a Magnetom Tim Trio 3-T scanner (Siemens Medical Solutions, Erlangen, Germany) at the Central Institute of Mental Health, Mannheim. A high-resolution T1-weighted three-dimensional image was acquired (slice thickness, 1.1 mm, field of view=256×240×176 mm3, matrix=256×240×160). Functional images were obtained from 40 gradient-echo T2*-weighted slices (slice thickness, 2.3 mm) per volume. We used a single-shot echo planar sequence with parallel imaging GRAPPA (generalized autocalibrating partially parallel acquisitions) technique (with an acceleration factor of 2)(TR=2,700 ms, flip angle=90°, TE=27 ms, field of view=220 mm2, matrix=96×96, slice gap=0.7 mm).
fMRI Data Analysis
Image processing and statistical analyses were performed with SPM8 (
http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Functional images were realigned, slice-time corrected, and spatially normalized using the Montreal Neurological Institute template. For normalization, images were resampled every 3 mm using sinc interpolation. Images were smoothed using a 9×9×9-mm Gaussian kernel.
Individual data were analyzed using a general linear model for blood-oxygen-level-dependent (BOLD) signal changes due to the experimental conditions. Movement parameters calculated during realignment were included as parameters of no interest to control for movement artifacts. Individual statistical parametric maps were calculated for the contrasts of interest in order to investigate BOLD signal changes in response to 1) mental arithmetic (task 1: arithmetic problems versus number detection) and 2) the influence of emotion on mental arithmetic (task 2: arithmetic problems superimposed on emotional versus neutral images).
Second-level random-effects analyses were conducted. First, one-sample t tests were calculated for the above-mentioned individual contrast images across participants in each of the three samples. Second, to evaluate differences between the bipolar patients, the at-risk populations, and their respective comparison counterparts, two-sample t tests were computed for all the contrasts. These analyses were not confounded by demographic differences because the groups were carefully matched. Third, to explore differences between the experimental groups directly, we drew a sample from all available healthy comparison subjects that was matched in size to the bipolar group (N=22) and matched in demographic variables as closely as possible to all three experimental groups. We then conducted analyses of variance (ANOVAs) with group as a between-subjects factor (the bipolar, relatives, hypomanic personality, and comparison groups) and age and gender as covariates, since the included groups differed significantly in demographic variables (see Table S1 in the
data supplement that accompanies the online edition of this article). Results of these analyses are reported in the
data supplement.
For all analyses, because direct contrasts of these conditions yielded no differences in the mental arithmetic task networks, we averaged across negative and positive stimuli and thus enhanced statistical power (
18,
35). Activations were thresholded at a whole-brain family-wise-error-corrected p value <0.05, with an extent threshold of 10 voxels in order to protect against false positive activations. From the activations found in task 1, a mask image was created. This mask was then used for task 2, in which activation was also thresholded with a family-wise-error-corrected p value <0.05 and a minimum of 10 voxels.
Behavioral Data Analysis
Reaction times and accuracy were analyzed with SPSS, version 20.0 (IBM, Armonk, N.Y.). Repeated-measures ANOVAs were calculated with group (bipolar patients compared with healthy comparison subjects, relatives compared with healthy comparison subjects, and hypomanic personality participants compared with healthy comparison subjects) as a between-subject factor and task (arithmetic, number detection), in Task 1, or emotion (emotional or neutral distractor), in Task 2, as a within-subject factor. As for the imaging data, additional analyses comparing all groups directly were carried out and included age and gender as covariates (see the online
data supplement).
Results
Behavioral Data
Task 1.
Accuracy was higher in the number detection task than in the arithmetic task (see Table S3 in the online
data supplement), which was evident in a main effect of task condition in each sample (sample 1: F=86.7, df=1, 42, p<0.001; sample 2: F=60.8, df=1, 32, p<0.001; sample 3: F=67.4, df=1, 42, p<0.001). There were no significant effects of group or interactions of group and task condition.
Regarding reaction times, there were significant main effects of task condition in each sample (sample 1: F=267.4.5, df=1, 41, p<0.001; sample 2: F=168.2, df=1, 32, p<0.001; sample 3: F=256.4, df=1, 42, p<0.001), with prolonged reactions in the arithmetic task compared with the number detection task, but no group effects were found.
Task 2.
For accuracy rates, we observed no significant main effects for distractor condition (emotional compared with neutral images) or group, nor a significant interaction effect (see Table S3 in the
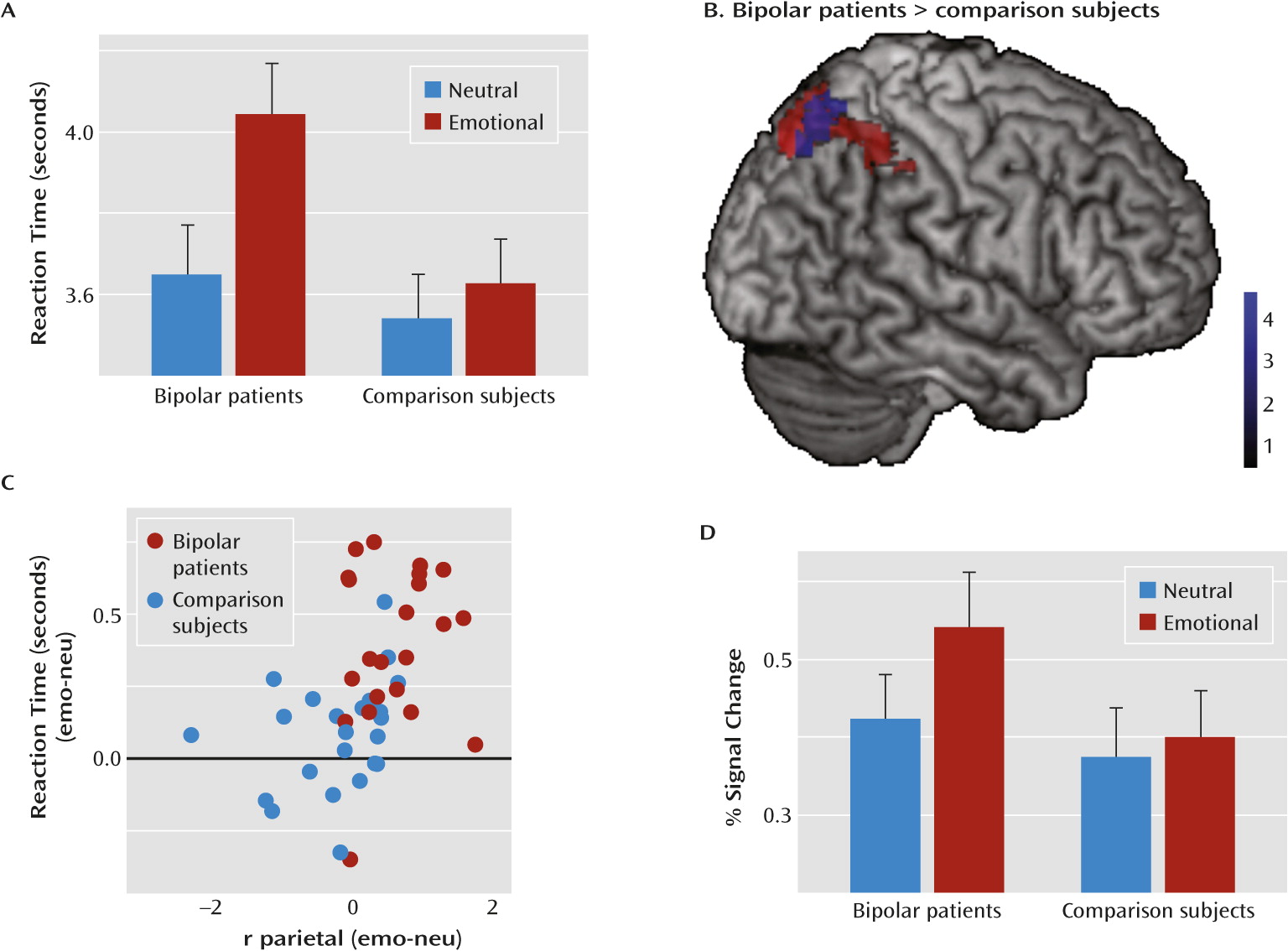
data supplement). However, participants were slower in solving arithmetic problems presented on emotional compared with neutral images, as evident in significant main effects of distractor condition on reaction times (sample 1: F=45.0, df=1, 42, p<0.001; sample 2: F=6.7, df=1, 32, p<0.05; sample 3: F=20.7, df=1, 42, p<0.001). We observed no significant main effect of group in any of the samples. While there were no interactions of group with the distractor condition in samples 2 and 3, there was an interaction in sample 1 with bipolar patients and healthy comparison subjects (F=18.7, df=1, 42, p<0.01). The difference between bipolar patients and their healthy counterparts was also larger than the difference between the other two groups and their respective comparison counterparts (Δ sample 1 compared with Δ sample 2: t=4.9, df=76, p<0.001; Δ sample 1 compared with Δ sample 3: t=6.4, df=88, p<0.001).
None of the behavioral effects correlated with medication load.
fMRI Data
Task 1.
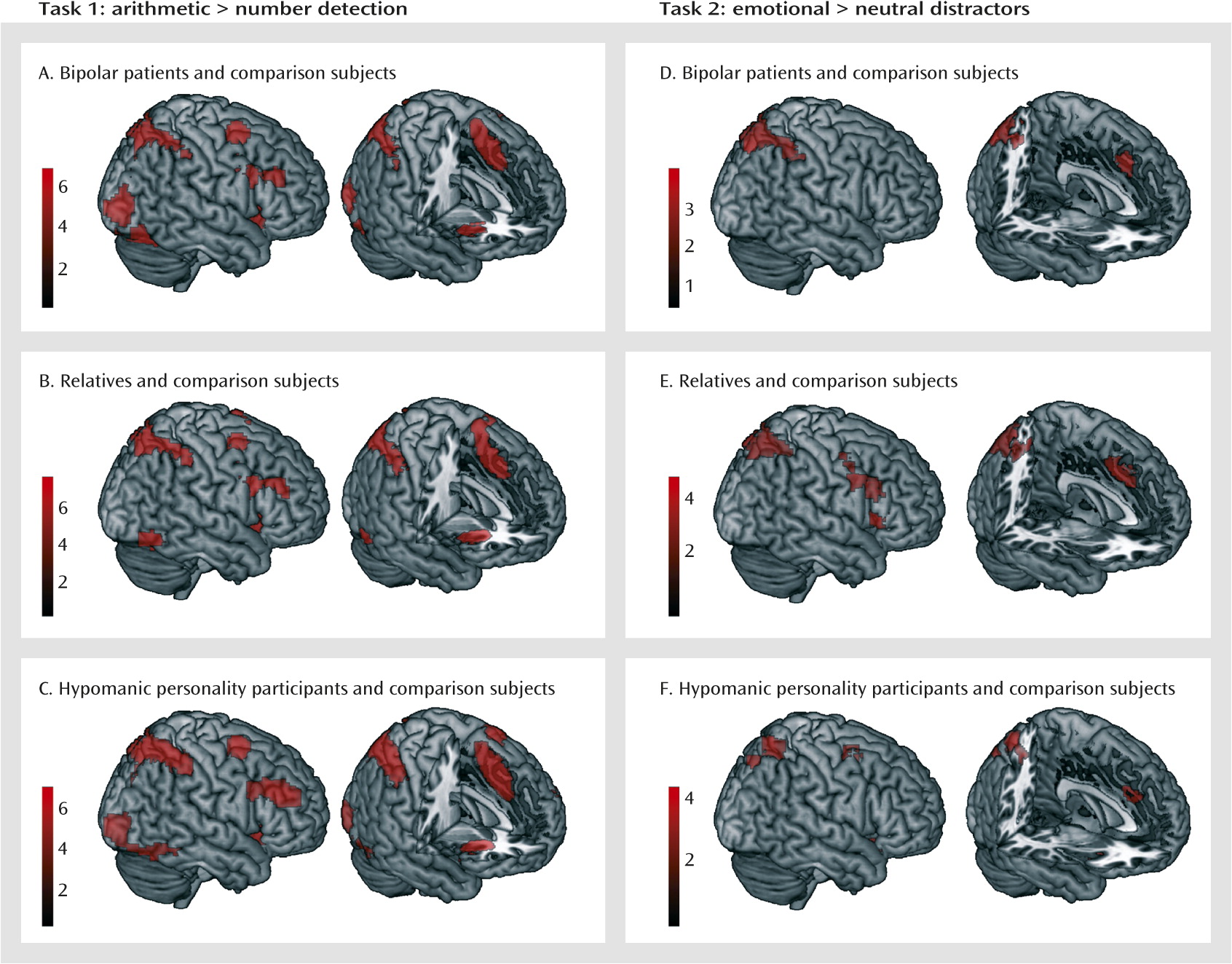
To elucidate the neural correlates of mental arithmetic, we contrasted the arithmetic task with the number detection task. There were no significant group differences in any of the samples. Therefore, we averaged across the groups in each sample (
Figure 2A–C; also see Table S4 in the
data supplement), which yielded largely consistent activation patterns. Activation foci included the left and right dorsolateral and dorsomedial prefrontal cortex, the insula, and the parietal cortex, extending into the occipital and ventral temporal cortex. Because the groups did not differ, one mask image for each sample with these networks was created for the analysis for task 2. There were no regions with enhanced activation in the number detection task.
Task 2.
To test the influence of emotional distractors on task processing, we contrasted the arithmetic task trials presented on emotional images with those presented on neutral images using the network identified in task 1 as a mask. For emotional background images, we found increased activation in the dorsolateral and dorsomedial prefrontal cortex and the parietal cortex in all three samples and in the left and right insula in samples 2 and 3 (
Table 2,
Figure 2D–F). There were no differences between relatives or individuals with hypomanic personality and their respective comparison counterparts. However, bipolar patients exhibited increased activation in the right parietal cortex compared with healthy comparison subjects (
Figure 3).
The difference in the extracted percent signal change in this region between bipolar patients and healthy comparison subjects was also larger than that between the other two groups and their respective comparison counterparts (Δ sample 1 compared with Δ sample 2: t=5.4, df=76, p<0.001; Δ sample 1 compared with Δ sample 3: t=4.5, df=88, p<0.001).
To relate the right parietal activation increase to the behavioral distraction effect, we correlated the reaction time increase with the activation increase (i.e., the differences between emotional and neutral trials). We found a significant correlation (r=0.45, p<0.01) (
Figure 3), which remained significant when calculating across all participants (r=0.32, p<0.005).
There were no regions with enhanced activation in response to neutral compared with emotional background images. For the bipolar patients, we also correlated the observed distraction effect with different clinical variables (i.e., the medication load, time in remission, age at illness onset, number of illness episodes, age at first hospitalization, and number of previous hospitalizations), but no significant correlations were observed. Additionally, there were no significant correlations with scores on the BDI, HAM-D, and Young Mania Rating Scale, with intelligence, or with years of education (see Table S8 in the
data supplement).
Discussion
The principal finding of this study is that euthymic bipolar patients experience exacerbated cognitive deficits under emotional distraction; this deficit is specific to bipolar patients in contrast to unaffected high-risk individuals. Bipolar patients exhibited increased response slowing during the arithmetic task while under emotional distraction, which was accompanied by a greater activation increase in a part of the task-related neural network, the right parietal cortex. That relatives of bipolar patients and participants with hypomanic personality were indistinguishable from their respective comparison counterparts suggests that this deficit is a consequence of the disorder rather than a vulnerability marker.
Neither bipolar patients nor high-risk individuals exhibited alterations in behavior or brain activity during the nonemotional mental arithmetic task. The identified neural network underlying mental arithmetic closely conforms with previous reports of clusters in the left and right parietal cortex (
19), the dorsolateral and dorsomedial prefrontal cortex (
20,
37), and the insula, a common finding in studies of arithmetic processing (
18,
38). The lack of group differences in behavioral and neural measures of task performance suggests that there are no general mathematical deficits in remitted bipolar patients and high-risk individuals. This allowed us to use the resulting activation patterns for the definition of one arithmetic task-specific neural network across groups.
When testing for the influence of emotional distractors on this network, bipolar patients exhibited prolonged response slowing. Consistent with our previous data from healthy individuals (
18), all participants needed longer to perform the task while under emotional compared with neutral distraction, but this effect was largely increased in the bipolar disorder group. The lack of any effect on error rates demonstrates that participants were still able to solve the equations and compensate for the distraction effect. The fMRI data mirrored this pattern. Task-related activation, particularly in the left and right parietal cortex, but also in the dorsolateral and dorsomedial prefrontal cortex, was increased in all participants under emotional distraction. Thus, it seems to be the additional recruitment of task-specific neural resources that enables individuals to compensate for emotional distraction effects, as has been previously suggested (
18,
39–
41). Critically, bipolar patients exhibited a larger interference effect than healthy comparison subjects, indicated by further enhanced activation increase in the right parietal cortex. Together with the increased response slowing, this suggests that bipolar patients are more affected by emotional distraction and need to recruit more task-specific neural resources to overcome the distraction effects. That is, those with the greatest distraction-related slowing exhibit the greatest degree of compensation to maintain accuracy in the face of slow performance. These results corroborate previous reports of increased activation in bipolar patients under emotional distraction in the absence of behavioral deficits (
11,
14–
16). The two-step fMRI procedure applied in our study, however, allows a clear interpretation of the activation increase as task specific, which is further supported by the correlation of increased response times with parietal hyperactivation.
Neither of the studied high-risk populations exhibited the increased emotional distraction effect observed in bipolar patients, although in previous studies, abnormal emotional processing (e.g., in responding to emotional faces) and emotion regulation have been observed in unaffected relatives (
42–
44) and in individuals with hypomanic personality (
45–
47). This selective impairment in remitted chronic bipolar patients suggests that increased emotional distractibility is a consequence of bipolar disorder that develops after the experience of at least one illness episode. Emotional stimuli are particularly salient distractors but seem to have an increased potential to impair cognition in bipolar disorder, for which an emotional hyperreactivity is well described. The persistent neuropsychological deficits after bipolar disorder onset are evidence of vulnerable cognitive processing. Our results suggest that this vulnerable system cannot manage emotional distraction as well as before illness onset. Interestingly, there was no correlation with current symptoms or clinical characteristics, such as the number of previous episodes, which may suggest that the deficit occurs after the first illness episode and remains stable thereafter. Future studies should investigate whether emotional distractibility is increased during acute episodes, whether it shows some valence specificity, and whether it increases with the number of experienced episodes. These studies should also compare first-onset and chronic bipolar patients.
Clinically, our findings are highly relevant with regard to patients’ functional recovery. Because emotional distraction leads to neuropsychological dysfunction, which in turn predicts functional recovery (
3,
4), interventions could include training of selective attention and emotion regulation, since this may be able to enhance sociofunctional integration in euthymic bipolar patients. Establishing such an intervention for improving neuropsychological performance in bipolar patients seems particularly important because these patients may attach emotional meaning even to nonemotional stimuli and tasks (
48–
50).
There are several limitations to this study. A large proportion of the tested patients continued to receive psychotropic medication. While it has been noted that this may increase generalizability (
33), it may also confound the results. We tested for an influence of medication by correlating the effects with a composite medication load score, which has previously been suggested and successfully applied (
33,
51–
53). However, future studies should include samples large enough to allow contrasting of drug subgroups and testing for effects in nonmedicated patients. It may also be possible that our sample sizes were not large enough to detect small differences; this is particularly relevant for the comparison of relatives and participants with hypomanic personality with their respective comparison counterparts. However, here the analysis was not only based on null effects in this comparison, but also on observed differences when comparing bipolar patients with any of the other groups (see the online
data supplement). Because approximately one-half of the relatives were >30 years old, one might argue that they do not represent a group at high risk but rather possess some resilience factor. However, we found no differences when directly comparing younger and older relatives (see the
data supplement), which corroborates the conclusion that the deficit develops only after the experience of an illness episode.
Conclusions
In summary, our results indicate increased emotional distractibility as a consequence of bipolar disorder. Hyperactivation in task-relevant neural regions is related to these behavioral deficits. The findings support a role of disturbed emotion-cognition interactions during the course of bipolar disorder that could critically hinder functional recovery and thus should be a specific target of treatment.
Acknowledgments
The authors thank Heike Schmidt for assistance with data acquisition.