Borderline personality disorder is a severe mental disorder characterized by emotional hyperarousal, anger proneness, and anger-related hostile, impulsive behaviors (
1). Borderline patients perceive ambiguous faces more negatively (
2) and exhibit stronger initial attention to and difficulties in disengaging attention from negative facial expressions (
3,
4). Regarding the perception of social threat, they tend to attribute resentment and aggression to others (
5) and are more likely to ascribe anger to ambiguous facial expressions (
6), favoring conflicts and reactive aggression. A bias toward negative or threatening information in borderline patients is also reflected in brain imaging data of increased and prolonged amygdala responses (
7–
9).
Initial eye movements provide a biologically relevant measure for the first reflexive orientation of visual attention to salient features of emotional stimuli (
10) and are sensitive to differences in social competences (
11,
12). However, they have not yet been investigated in borderline patients. Previous studies of healthy volunteers suggest that the ventral parts of the posterior amygdala, most likely the accessory basal nucleus, play an important role in initial reflexive attentional shifts toward the eye region (
10,
13), the most salient and arousing feature of threatening facial expressions (
14). Thus, although eye contact is important for social functioning, exaggerated initial fixation changes toward the eyes of threatening facial expressions may be related to emotional hyperarousal and could lead to impulsive, hostile behaviors.
The neuropeptide oxytocin is involved in social behavior across species. In healthy individuals, the intranasal administration of oxytocin reduces anxiety and stress in social situations (
15), enhances the recognition of facial expressions (
16–
19), and shifts attention from negative to positive information (
20–
22), although individual differences and situational factors seem to play an important role (
23). Neuroimaging and animal studies indicate that oxytocinergic modulation of social behavior is related to its effects on the amygdala (
24). In healthy men, oxytocin administration reduces activity in the (dorsal and lateral anterior) amygdala to negative emotional stimuli (
13,
25,
26), which may reflect a neural mechanism of its anxiolytic properties (
24,
26). However, contrary effects are reported in healthy women (
27,
28). In addition, oxytocin administration increases initial, reflexive fixation changes toward the eyes of faces in association with increased activity in the posterior amygdala (
13). Taken together, oxytocin may be a powerful modulator of the salience of social information (
23) optimizing reflexive processing of social cues, as reflected in amygdala activation and initial eye movements. It has therefore been suggested that borderline patients who are hypersensitive to negative, threatening social information may benefit from intranasal oxytocin administration (
29). Consistent with this assumption, oxytocin was shown to reduce social stress (
30) in borderline patients, although it did not increase trust and cooperation during a game paradigm (
31).
The aim of the present study was to investigate oxytocinergic effects on facial threat processing in borderline patients. We intranasally administered 26 IU of oxytocin or a placebo in a double-blind design to women with and without borderline personality disorder. We compared the number and duration of initial eye movements, manual response latencies, and amygdala activation between groups by combining eye tracking and functional MRI (fMRI). Since we were interested in oxytocin as a modulator of the salience of threatening social cues, we selected high-resolution fMRI (2×2×2 mm
3) focusing on the amygdala, including its subregions, which have been shown to be differentially modulated by oxytocin (
13). We expected borderline patients to exhibit more and faster fixation changes to the eyes of angry faces and enhanced amygdala activation in response to angry faces. We hypothesized that oxytocin would decrease the number and speed of fixation changes and reduce activation of the amygdala, or more specifically of the posterior amygdala, in borderline patients in response to angry faces. In line with previous findings (
27,
28), we expected oxytocin to increase amygdala activation in healthy women in response to negative faces.
Method
Participants
Participants were 40 female patients with a diagnosis of borderline personality disorder, as defined by DSM-IV criteria, and 41 healthy women between 18 and 36 years old (mean age, 24.4 years [SD=4.7]). Exclusion criteria were an IQ ≤85; pregnancy; endocrine or neurological disorders; use of any type of regular medication except contraceptives; lifetime diagnoses of schizophrenia, schizoaffective disorder, or bipolar disorder; and current alcohol or drug dependence. Further exclusion criteria for healthy control subjects were current or past psychiatric diagnoses and psychological or psychiatric treatment. Borderline patients were recruited at the Department of General Psychiatry at the University of Heidelberg (N=12) and through advertisement (N=28). Healthy women were recruited through advertisement, were matched with patients on age, IQ, and education, and underwent the same diagnostic procedure as patients.
All experiments were performed in the early follicular phase (days 2–7 after onset of menses) as assessed by participants’ self-report and validated by blood assays to exclude possible interactions of exogenous oxytocin and fluctuations of gonadal steroids over the menstrual cycle. Progesterone and estradiol values confirmed that all participants were in the follicular phase (progesterone level <2.0 ng/ml, estradiol level <165 pg/ml) except two borderline patients (one from the oxytocin condition and one from the placebo condition), who were excluded from further analyses. In addition, data for another 13 women were discarded from the analyses of the behavioral data because of artifacts, resulting in an insufficient number of trials per condition. Thus, analyses of behavioral data were based on 66 participants (19 borderline patients in the oxytocin condition,16 borderline patients in the placebo condition, 15 healthy women in the oxytocin condition, and 16 healthy women in the placebo condition; mean age, 24.6 years [SD=5.0]), while analyses of fMRI data were based on 79 participants (19 borderline patients in the oxytocin condition, 19 borderline patients in the placebo condition, 21 healthy women in the oxytocin condition, and 20 healthy women in the placebo condition; mean age, 24.3 years [SD=4.8]).
The study was approved by the Ethics Committee of the Heidelberg University Faculty of Medicine. All participants provided written informed consent and were financially compensated for their participation.
Measures
Qualified diagnosticians assessed borderline diagnosis and comorbidities with axis II (International Personality Disorder Examination for DSM-IV [
32]) and axis I (Structured Clinical Interview for DSM-IV) disorders before the experiment (
Table 1). Four subtests of the Hamburg-Wechsler Intelligence Test provided an estimate of intelligence, and borderline symptom severity and depressiveness were assessed with the Borderline Symptom List−23 (
33) and the Beck Depression Inventory.
Experimental Protocol
Participants were instructed to abstain from smoking, caffeine, and analgesic medication on the day of the experiment and from eating 2 hours before the experiment. In a double-blind placebo-controlled design, participants were assigned to either the oxytocin condition, in which a single intranasal dose of 26 IU of oxytocin was administered (five sprays, each with 5.2 IU of oxytocin), or the placebo condition, in which a placebo spray containing the same inactive ingredients as the active spray, but no oxytocin, was administered. Because central nervous oxytocin levels reach a plateau approximately 40 minutes after substance administration (
34), participants received oxytocin or placebo 45 minutes before fMRI scanning (scan duration, <45 minutes). Potential nonspecific effects of oxytocin on mood, wakefulness, or arousal were assessed with a multidimensional mood questionnaire (see the
data supplement that accompanies the online edition of this article).
Emotion Classification Paradigm
The task followed a 3×2 design, with the within-subject factors of emotional expression (angry, fearful, and happy) and initial fixation (eyes, mouth), and allowed the investigation of reflexive gaze behavior in response to direct eye contact or initial fixation on the mouth (
10,
13). In each of three experimental sessions, participants had to classify 72 briefly presented faces, unambiguously depicting angry, fearful, or happy expressions (12 male and 12 female faces for each expression in a randomized sequence; see also the online
data supplement), by pressing a corresponding key as quickly and accurately as possible. One-half of the faces within each emotional expression were unpredictably shifted downward or upward to investigate the effect of initial fixation. Each trial started with a fixation cross (2 seconds) followed by the presentation of a face with the eyes or the mouth appearing at the location of the formerly presented fixation cross (150 ms), a blank screen (1,850 ms), and another fixation cross (2–9 seconds).
Data Acquisition
Blood-oxygen-level-dependent functional images and behavioral data were acquired throughout the experiment. Faces were presented using Presentation software, version 14.2 (Neurobehavioral Systems, Albany, Calif.), through video projection and a special mirror mounted to the head coil. Gazing behavior was measured with a 60-Hz MRI-compatible eye-tracking camera (MRC Systems, Heidelberg, Germany) mounted laterally underneath the participant’s right eye.
Functional imaging was performed on a Tim Trio 3-T whole-body MR scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with a 32-channel head coil. Thirty-five transverse slices (slice thickness, 2 mm; no gap) were acquired in each volume covering the temporal lobe and occipital and orbitofrontal cortex to achieve high anatomical resolution of amygdala subregions, which may be differentially modulated by oxytocin (
13). A T
2*-sensitive gradient echo-planar imaging sequence was used (TR=2,260 ms; TE=30 ms; flip angle=80°; field of view=208×208 mm; in-plane resolution=2×2 mm). Additionally, isotropic high-resolution (1×1×1 mm
3) structural images were recorded using a T
1-weighted coronal-oriented magnetization-prepared rapid gradient echo sequence.
Data Analysis
Behavioral data.
Details on the analyses of eye movements are presented in the online
data supplement. Briefly, we determined 1) the latencies and 2) the proportion of fixation changes (>0.5°) toward the other major facial feature that were triggered by the stimulus but occurred after stimulus offset (
10,
13). Hence, when the eyes were presented at the position of the fixation cross, we determined the latency and proportion of downward fixation changes toward the mouth, and when the mouth followed the fixation cross, we calculated the corresponding upward fixation changes toward the eyes. Next, we determined response latencies and then the proportion of correct responses.
Data were analyzed with analyses of variance using group (borderline patients, control subjects) and condition (oxytocin, placebo) as group factors, and initial fixation (eyes, mouth) and emotion (angry, fearful, happy) as within-subject factors. Where appropriate, the Huynh-Feldt procedure was applied to correct for potential violations of the sphericity assumption. All statistical analyses employed a two-tailed p value <0.05. In cases of significant effects, we used Dunn’s multiple comparison as post hoc tests.
fMRI data.
Statistical parametric mapping with SPM8 (
http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) was used for preprocessing and analyzing the imaging data. A 4-mm full-width-at-half-maximum isotropic Gaussian smoothing kernel was used to optimize the detection of small activation foci within the amygdala (see the online
data supplement).
To examine changes in brain activation as a function of the experimental manipulation, we constructed a design matrix for each participant by modeling the onset of the face presentation (convolved with the hemodynamic response function) as separate regressors for all six combinations of emotion (angry, fearful, happy) and initial fixation (eyes, mouth) in each session. Individual contrast maps (each emotion-by-initial fixation combination) were then subjected to a second-level random effects analysis. Within this model, we compared brain activation between angry and happy faces and between fearful and happy faces in the four groups (borderline patients in the oxytocin or placebo condition and control subjects in the oxytocin or placebo condition) and estimated interaction contrasts to test for effects of group (borderline patients compared with control subjects) and condition (oxytocin compared with placebo). Since we hypothesized modulatory effects of oxytocin in the amygdala, we applied a small-volume correction for multiple comparisons in predefined bilateral anatomical amygdala regions of interest (
35). The threshold for all tests was set at a p value <0.05 (family-wise-error corrected). All significant activations are reported using Montreal Neurological Institute space. Anatomical labels for subregions within the amygdala were specified by comparing the location of activation clusters with high-resolution diagrams of the human amygdala as depicted in an anatomical atlas (
36).
All data analyses were repeated controlling for depressiveness, mood, wakefulness, and arousal; this did not influence the results.
Results
Behavioral Data
Proportion of correct responses.
Participants were highly accurate in classifying the displayed facial emotions, and the four groups did not differ significantly in classification accuracy (see Table S1 in the online
data supplement). Across conditions, participants were better at classifying happy faces than angry and fearful faces (F=35.46, df=2, 124, p<0.001) and better at classifying faces that were presented with the eyes at the position of the fixation cross (F=5.39, df=1, 62, p=0.02). There were no differences between borderline patients and control subjects.
Response latencies.
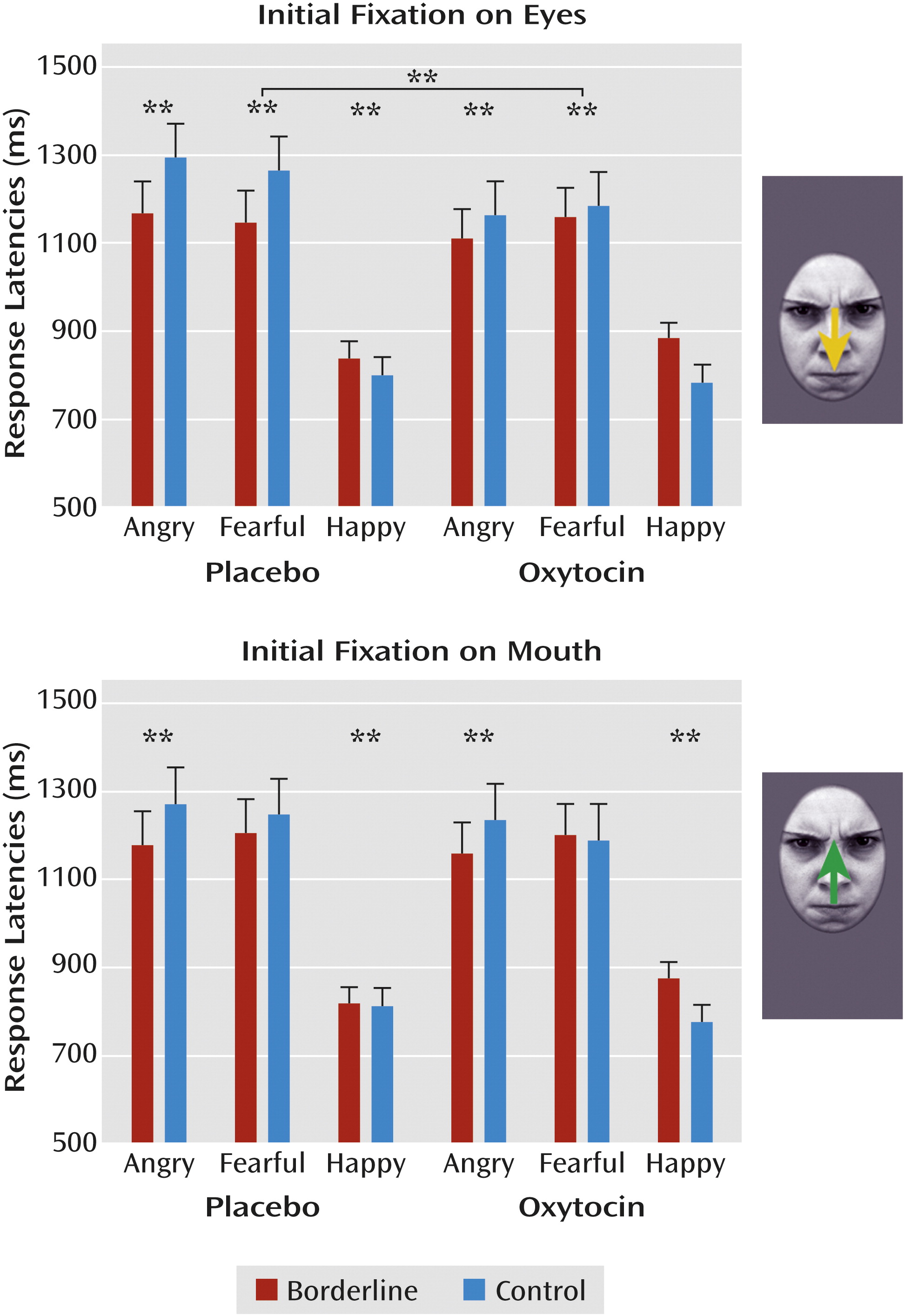
Overall, participants responded faster to happy compared with angry and fearful faces (F=126.12, df=2, 124, p<0.001), but borderline patients responded faster to angry faces than participants in the control group (F=3.94, df=2, 124, p=0.03). Additionally, a significant interaction of group, fixation, and emotion (F=3.24, df=2, 124, p<0.05) indicated that compared with control subjects, borderline patients responded faster to angry faces with initial fixation on the eye (p<0.01) and mouth region (p<0.01) and to fearful faces with initial fixation on the eye region (p<0.01) but slower to happy faces (p<0.01). Analysis of a condition, fixation, and emotion interaction (F=3.58, df=2, 124, p=0.03) revealed that borderline patients and healthy women in the oxytocin condition reacted faster to angry faces with initial fixation on the eye region than participants in the placebo groups (p<0.01). Descriptively, this latter effect seemed to mainly depend on differences in response latencies between healthy women receiving oxytocin compared with placebo (
Figure 1), but the four-way interaction did not reach statistical significance.
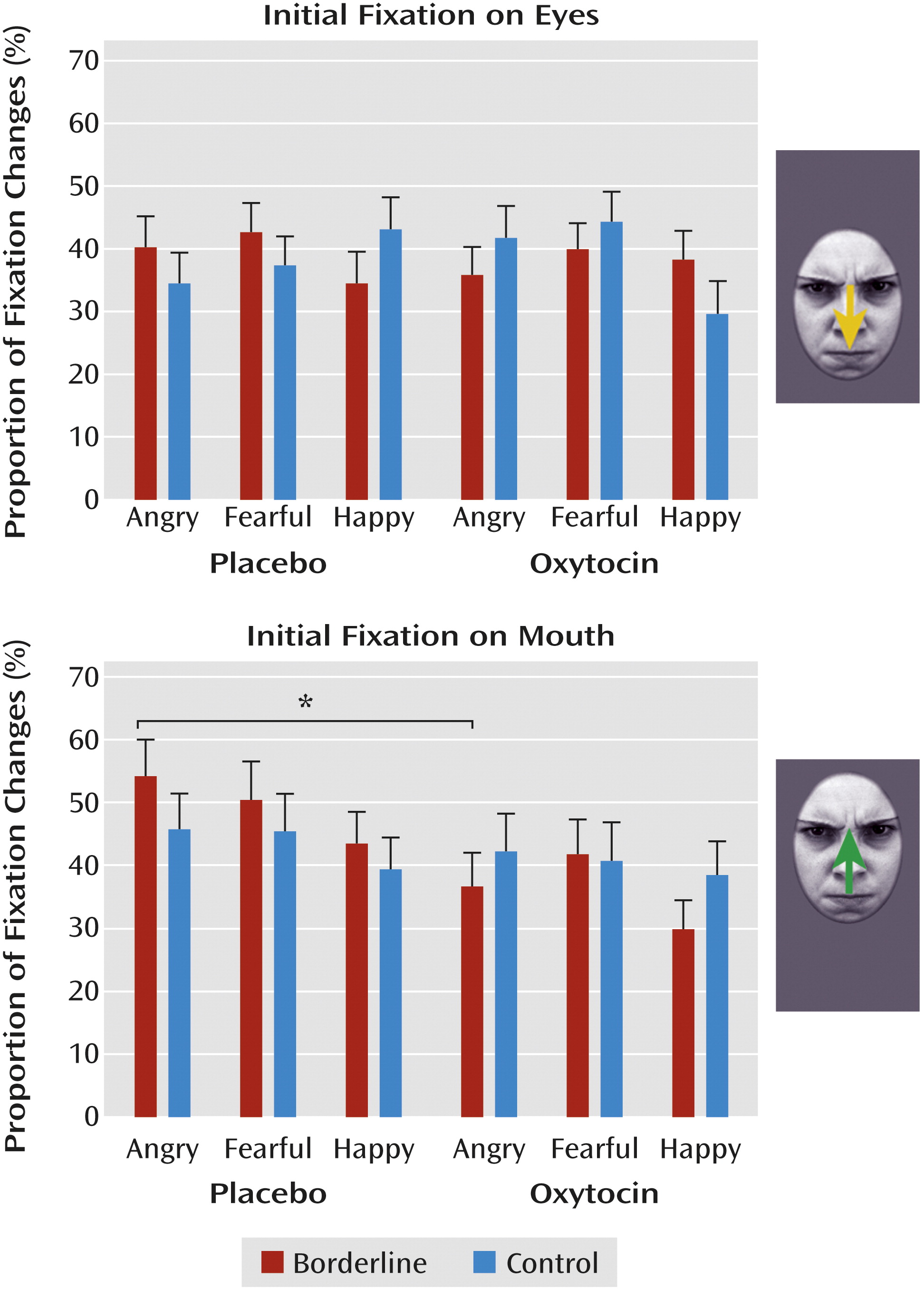
Proportion of fixation changes.
Across conditions, participants made more fixation changes to angry and fearful faces than to happy faces (F=7.36, df=2, 124, p=0.001). Additionally, we observed a significant four-way interaction of group, condition, fixation, and emotion (F=4.02, df=1, 124, p=0.02), indicating that borderline patients in the placebo condition made more reflexive fixation changes to the eyes of angry faces than patients in the oxytocin condition (p<0.01;
Figure 2). Thus, in borderline patients, oxytocin seemed to dampen the likelihood of fixation changes toward the eyes of angry faces.
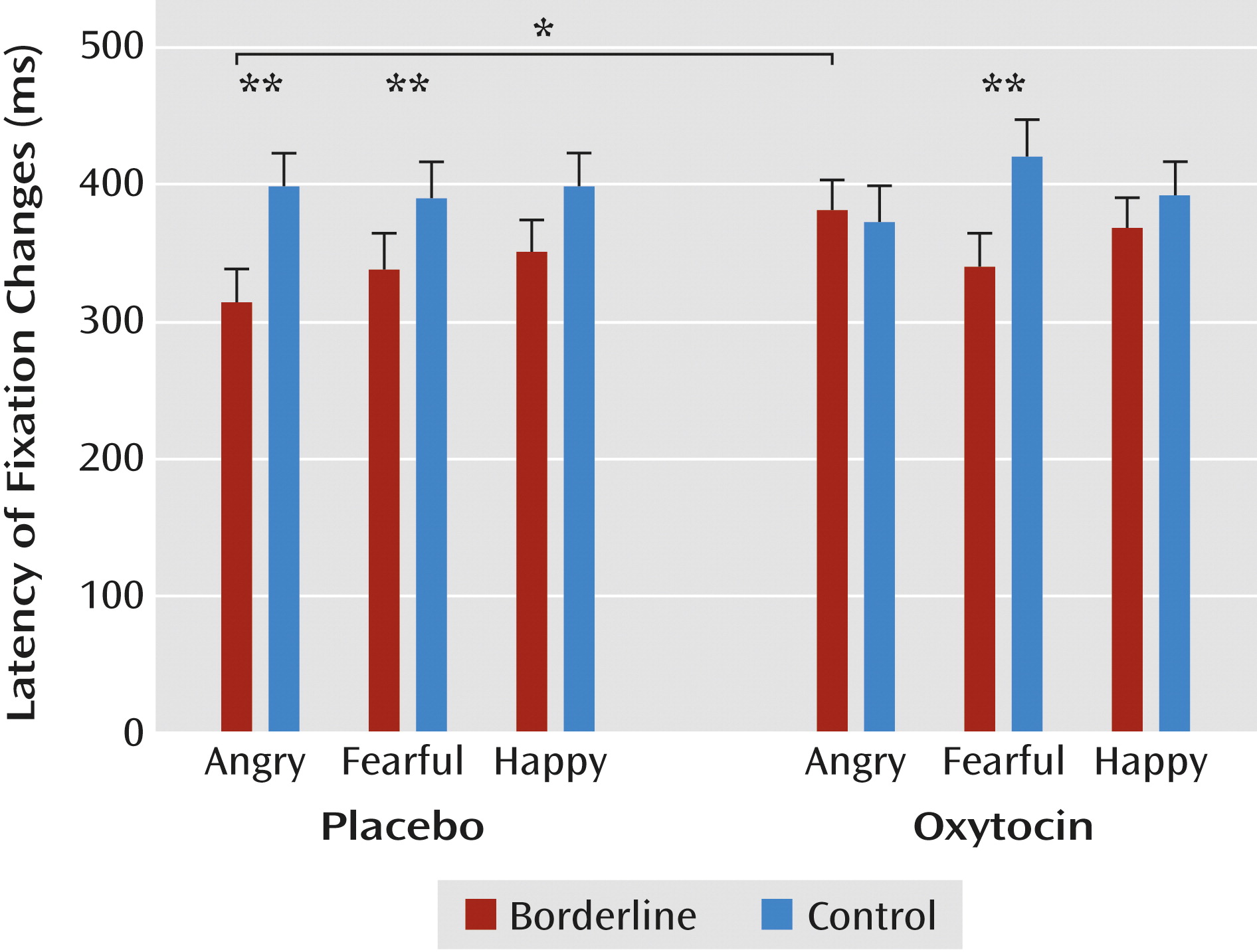
Latency of fixation changes.
Borderline patients made faster fixation changes than participants in the control group (F=4.61, df=1, 62, p=0.04). A significant interaction of initial fixation and emotion (F=3.32, df=2, 124, p=0.04) also indicated generally faster fixation changes to the eyes than to the mouth of angry (p<0.01) and fearful (p<0.01) faces. A significant three-way interaction of group, condition, and emotion (F=3.32, df=2, 124, p=0.04) revealed faster fixation changes among borderline patients in the placebo condition for angry faces, both compared with healthy women (p<0.01) and with borderline patients in the oxytocin condition (p<0.05;
Figure 3). Thus, in borderline patients, oxytocin seemed to dampen the enhanced speed of initial fixation changes to angry faces.
Imaging Data
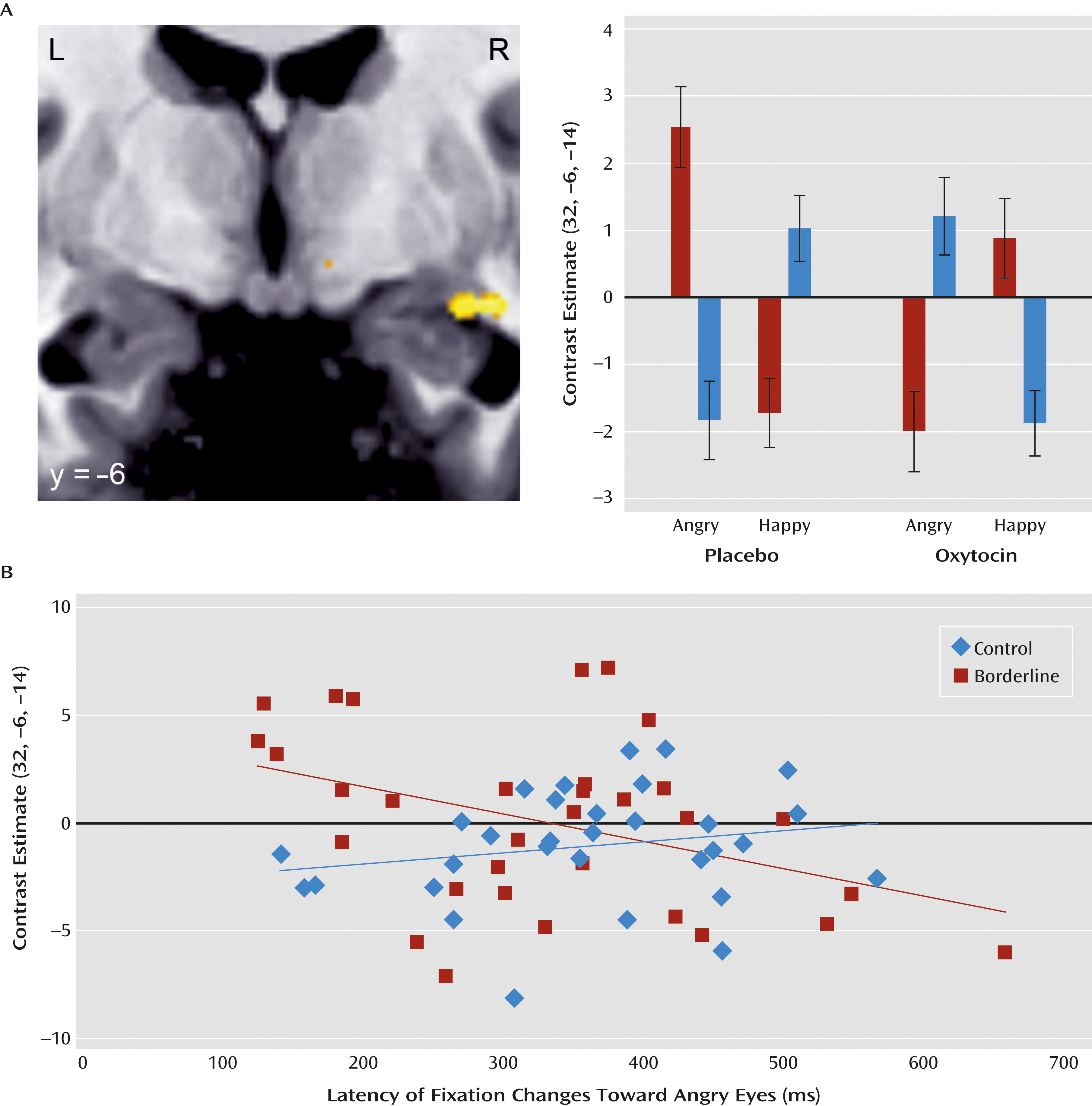
As expected, borderline patients in the placebo condition exhibited stronger amygdala activation in response to angry and fearful faces compared with happy faces (fearful versus happy: peak voxel [x, y, z]: −19, −5, −19; z=3.18, p=0.07, family-wise-error corrected; angry versus happy: peak voxel [x, y, z]: 32, −6, −15; z=3.63, p=0.02, family-wise-error corrected). Effects were strongest for angry expressions, particularly those with initial fixation on the eye region. Except for this latter contrast (angry versus happy with initial fixation on the eyes: peak voxel [x, y, z]: −29, −7, −18; z=3.35, p<0.05, family-wise-error corrected), patients in the oxytocin condition did not exhibit significantly enhanced amygdala activation in response to angry or fearful faces compared with happy faces. The direct comparison between the two borderline groups (placebo versus oxytocin for angry versus happy and fearful versus happy) revealed reduced activity in the right posterior amygdala in patients in the oxytocin condition contrasted with patients in the placebo condition in response to angry compared with happy faces (peak voxel [x, y, z]: 30, −6, −14; z=3.38, p<0.05, family-wise-error corrected), but no significant group difference for fearful compared with happy faces was observed.
Equivalent analyses in healthy participants revealed enhanced activation in the right posterior amygdala in response to angry faces with initial fixation on the mouth region in participants in the placebo condition (peak voxel [x, y, z]: 24, −6, −17; z=3.59, p=0.02, family-wise-error corrected) but enhanced right, more centrally located amygdala activation in response to fearful faces with initial fixation on the eye region in those in the oxytocin condition (peak voxel [x, y, z]: 28, −3, −18; z=3.75, p=0.01, family-wise-error corrected).
Finally, direct comparisons of women with and without borderline personality disorder (placebo versus oxytocin for borderline group versus control group for angry versus happy and fearful versus happy) revealed a marginally significant effect in the right amygdala in response to angry compared with happy faces (peak voxel [x, y, z]: 32, −6, −14; z=3.15, p=0.09, family-wise-error corrected). Thus, oxytocin tended to differentially modulate the activation in a cluster located in the right posterior amygdala in borderline patients and control subjects: While increasing the activity in response to angry compared with happy faces in healthy women, oxytocin reversed the increased activation in response to angry compared with happy faces in borderline patients (
Figure 4A). Importantly, the reverse comparisons did not reveal any significant effect in the amygdala.
Additional correlation analyses revealed that faster fixation changes to the eyes of angry faces were related to increased right posterior amygdala responses in borderline patients (r=−0.40, p<0.05) but not in control subjects (
Figure 4B).
Discussion
This is the first study, to our knowledge, to reveal beneficial effects of intranasally administered oxytocin on facial threat processing in female borderline patients. By combining eye tracking with high-resolution fMRI, we found a reduction of posterior amygdala hyperactivation, which was related to a reduced attentional bias toward socially threatening cues (i.e., the eyes of angry faces) after oxytocin administration in borderline patients.
Compared with healthy women, borderline patients in the placebo condition were faster at recognizing anger and, if initially fixating on the eyes, fear in briefly presented faces, supporting previous reported enhanced detection of subtle facial threat (
2,
6), which may be a result of increased arousal in borderline patients (
37). This is further endorsed by the pattern of reflexive fixation changes in borderline patients, with faster eye movements in response to angry and fearful faces and more initial fixation changes toward the eyes of angry faces compared with healthy individuals. Independent of valence, the eye region is regarded as the most salient and arousing facial feature (
14), capturing early orientation and constituting the experienced intensity of facial expressions (
10).
In borderline patients, the exaggerated initial fixation changes toward the eyes of angry faces were related to enhanced activity in the posterior amygdala and thus may reflect a fast, reflexive hyperreactivity to social threat in borderline personality disorder. Supporting the hypothesis of an early attentional bias for social threat (
37), this may have led to faster manual responses to briefly presented angry (and fearful) faces in borderline patients in the placebo condition, compared with control subjects. In addition, when initially fixating on the eyes of threatening faces, borderline patients in the placebo condition also exhibited earlier fixation changes away from the eye region, again combined with increased amygdala activity, supporting the hypothesis that excessive emotional arousal mediates the avoidance of the eyes (
11). Thus, although eye contact is important for social functioning, rapid and exaggerated eye movements toward the eyes of angry faces seem to be related to emotional hyperarousal in borderline personality disorder, and hence one of the key characteristics of this disorder that often leads to impulsive, self-injurious, or aggressive behaviors (
1).
Regarding the oxytocin effects, we found increased activity in a more centrally located amygdala region, most likely belonging to the corticomedial nuclear group, in healthy women when directly confronted with the eyes of fearful faces, thus replicating previous findings in the midluteal phase of females (
27,
28) in the early follicular phase. This contradicts the anxiolytic-like oxytocinergic effects, including a dampening of activity in the corticomedial and lateral nuclei of the anterior amygdala, found in healthy men in response to fearful faces (
13). Although oxytocin improved the detection of facial anger in healthy women in our study in terms of faster manual reactions, we found neither an increased number of reflexive gaze changes toward the eyes of angry faces nor increased posterior amygdala activity in response to angry faces that were initially fixated on the mouth. This contrasts sharply with the previous study of healthy men (
13), calling for a direct comparison of male and female participants in future studies, while strengthening the hypothesized gender- and trait-dependent oxytocinergic modulation of social behavior (
23).
In female borderline patients, oxytocin reduced the exaggerated speed and number of first, reflexive eye movements in response to the eyes of angry faces and dampened the often-reported amygdala hyperreactivity to threatening information (
7–
9). Oxytocin particularly affected activations in the posterior part of the amygdala, presumably corresponding to the basal nucleus. This region seems to be involved in the assignment of salience to negative facial expressions in the visual periphery and in redirecting attention toward socially relevant locations in the visual field (
10,
38). Our results, therefore, do not support a general oxytocinergic enhancement (
13) but rather indicate a trait-dependent modulation of social salience, which may help to optimize an individual’s social behavior (
23). Similarly, oxytocin has been found to reduce amygdala activation and threat biases, particularly in those participants with enhanced reactivity to negative emotional stimuli (e.g., individuals with high depressiveness [
21] or hostility [
22]). Contrary to this, oxytocin improves emotion recognition, social behavior, and eye contact and, on the neuronal level, enhances amygdala activation in individuals with low emotional sensibility (
17) or autism spectrum disorder (
39,
40). In emotionally hyperaroused borderline patients, who exhibit exaggerated allocation of attention toward the most threatening features of social information, a down-regulation of social salience by oxytocin may help to reduce stress reactivity (
30), hostility, and reactive aggressive behaviors. However, oxytocinergic effects in borderline patients may differ as a function of traumatization and attachment security as demonstrated by Bartz et al. (
31). In that study, oxytocin promoted cooperation in low-avoidant individuals but impeded cooperation in intimacy-avoidant individuals. Thus, future studies should address differential oxytocinergic modulation of social cognition in subtypes of borderline patients and disentangle an apparently complex relationship of childhood adversity, attachment, and oxytocin in borderline personality disorder (
41).
Our study has several strengths, displaying early, reflexive mechanisms of biased threat processing and beneficial effects of oxytocin administration in borderline patients using a combination of eye tracking and high-resolution fMRI. However, several limitations should be noted. First, diagnostic interviews revealed a number of comorbidities in the patient sample, which reflects a typical pattern of comorbid psychiatric disorders in young, female borderline patients (
42). Hypersensitivity to social threat, which has been replicated in several different samples of borderline patients, and oxytocinergic effects were not altered after controlling for depressiveness. Second, because of the lack of a clinical control group, borderline-specific conclusions should be drawn with caution. Third, high-resolution fMRI with focus on the amygdala did not allow for measuring whole-brain activation changes. Future studies employing whole-brain fMRI would be helpful to analyze oxytocinergic modulations in other regions involved in emotional processing. An exploratory analysis of the whole volume did not reveal main or interaction effects at a p value <0.001 (uncorrected). Additional region-of-interest analyses of functionally (insula) and locally (hippocampus) related regions did not reveal similar patterns of effects, underlining the specificity of the reported amygdala activations. Fourth, behavioral data of 13 participants had to be excluded because of artifacts, and the number of valid eye movements was smaller than that found by Gamer et al. (
10,
13), probably because of differences in technical characteristics of the eye tracker. Importantly, borderline patients and healthy control subjects did not differ in the number of valid trials. In addition, the remarkable spatial overlap of amygdala regions related to eye movements indicates an involvement of similar processes across studies (
10,
13). Finally, the sample size is limited, although it is comparable to most previous studies of this sort (
9,
13,
25–
28).
Taken together, this study revealed a reduced attentional bias toward social threat in terms of reduced speed and number of reflexive eye movements toward the eyes of angry faces and attenuated amygdala activations in response to angry faces compared with happy faces following oxytocin administration in female borderline patients. These results shed initial light on the beneficial effects oxytocin may exert in borderline or other psychiatric populations with enhanced threat-driven reactive aggression.