Internet-Delivered Treatment for Substance Abuse: A Multisite Randomized Controlled Trial
Abstract
Objective
Method
Results
Conclusions
Method
Recruitment Sites
Study Design
Participants
Internet-Delivered Intervention
Assessments
Sample Size, Power, and Statistical Analysis
Results
| Variable | Overall Sample (N=507) | Treatment as Usual Plus TES (N=255) | Treatment as Usual (N=252) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 34.9 | 10.9 | 35.6 | 10.7 | 34.2 | 11.1 |
| Days of alcohol or drug use in past 30 days | 9.8 | 9.4 | 10.2 | 8.9 | 9.4 | 9.8 |
| N | % | N | % | N | % | |
| Femaleb | 192 | 37.9 | 91 | 35.7 | 101 | 40.1 |
| Racec | ||||||
| White | 284 | 56.0 | 136 | 53.3 | 148 | 58.7 |
| Black or African American | 116 | 22.9 | 69 | 27.1 | 47 | 18.7 |
| American Indian or Alaska Native | 3 | 0.6 | 2 | 0.8 | 1 | 0.4 |
| Asian | 13 | 2.6 | 6 | 2.4 | 7 | 2.8 |
| Native Hawaiian or Pacific Islander | 12 | 2.4 | 7 | 2.7 | 5 | 2.0 |
| Multiracial | 54 | 10.7 | 23 | 9.0 | 31 | 12.3 |
| Other | 23 | 4.5 | 10 | 3.9 | 13 | 5.2 |
| Hispanic or Latinod | 55 | 10.8 | 26 | 10.2 | 29 | 11.5 |
| Education | ||||||
| <High school diploma | 118 | 23.3 | 60 | 23.5 | 58 | 23.0 |
| High school diploma or GED | 310 | 61.1 | 161 | 63.1 | 149 | 59.1 |
| >High school diploma | 79 | 15.6 | 34 | 13.3 | 45 | 17.9 |
| Marital status | ||||||
| Never married | 308 | 60.7 | 148 | 58.0 | 160 | 63.5 |
| Married or remarried | 72 | 14.2 | 36 | 14.1 | 36 | 14.3 |
| Separated, divorced, or widowed | 127 | 25.0 | 71 | 27.8 | 56 | 22.2 |
| Underemployed (unemployed/irregular part-time) | 190 | 37.5 | 106 | 41.6 | 84 | 33.3 |
| Primary substance | ||||||
| Alcohol | 104 | 20.5 | 58 | 22.7 | 46 | 18.3 |
| Cocaine | 102 | 20.1 | 53 | 20.8 | 49 | 19.4 |
| Stimulants | 69 | 13.6 | 33 | 12.9 | 36 | 14.3 |
| Marijuana | 114 | 22.5 | 54 | 21.2 | 60 | 23.8 |
| Opiates | 108 | 21.3 | 49 | 19.2 | 59 | 23.4 |
| Other | 10 | 2.0 | 8 | 3.1 | 2 | 0.8 |
| Substance dependencee | ||||||
| Alcohol | 224 | 44.2 | 119 | 46.7 | 105 | 41.7 |
| Cocaine | 177 | 34.9 | 90 | 35.3 | 87 | 34.5 |
| Stimulants | 100 | 19.7 | 47 | 18.4 | 53 | 21.0 |
| Marijuana | 146 | 28.8 | 68 | 26.7 | 78 | 31.0 |
| Opiates | 158 | 31.2 | 78 | 30.6 | 80 | 31.7 |
| Other | 41 | 8.1 | 21 | 8.2 | 20 | 7.9 |
| Abstinent at baseline and study entry | 275 | 54.2 | 136 | 53.3 | 139 | 55.2 |
Treatment Adherence
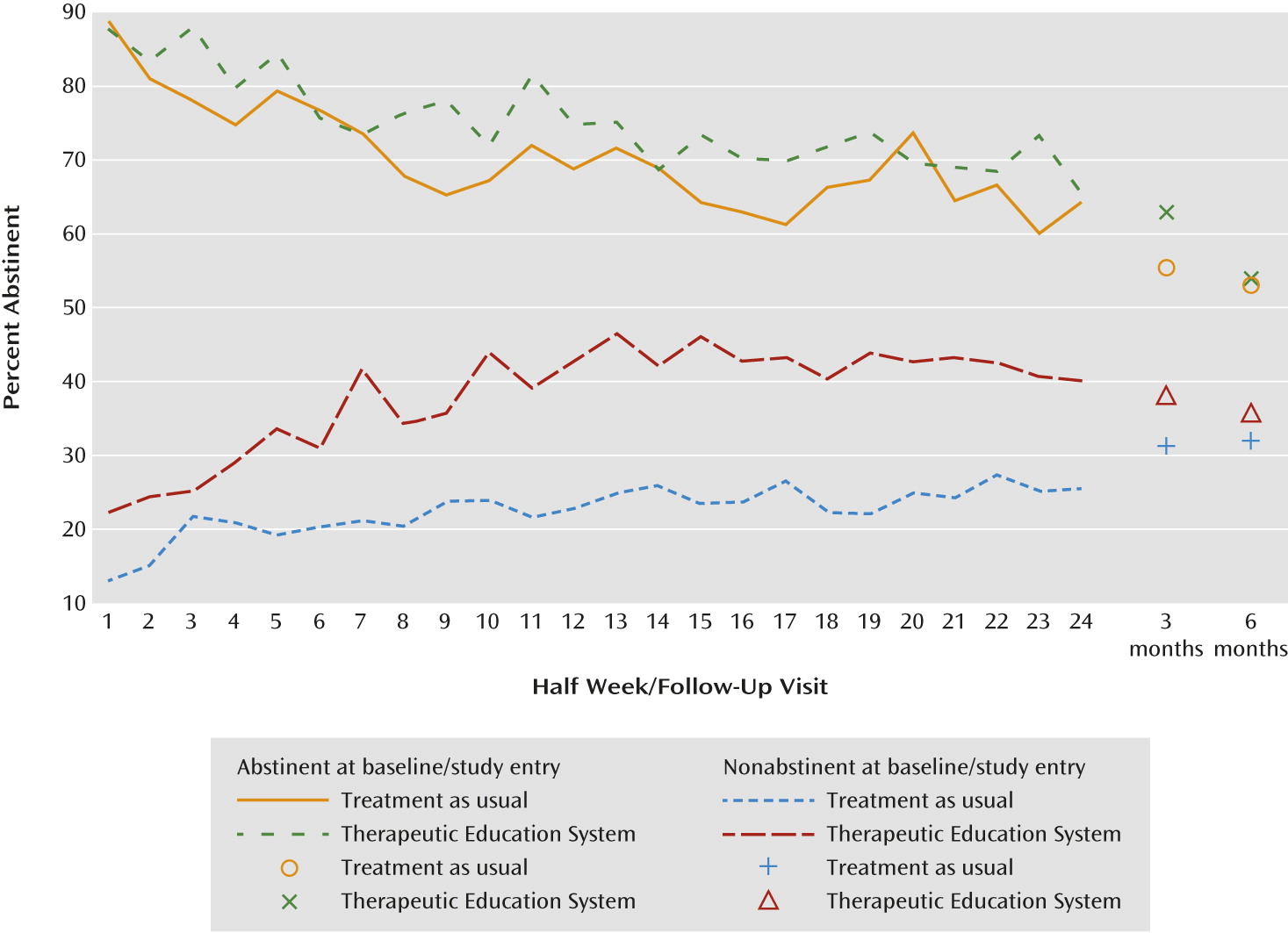
Effect of Treatment on Abstinence
| Model and Variable | Odds Ratioa | 95% CI | p |
|---|---|---|---|
| Model 1: Main effects | |||
| Abstinent at baseline/study entry | 5.73 | 4.20, 7.80 | <0.001 |
| Stimulant as primary substance | 1.23 | 0.90, 1.68 | 0.193 |
| Clinical siteb | 0.003 | ||
| Treatment (TES versus treatment as usual) | 1.62 | 1.12, 2.35 | 0.010 |
| Model 2c: Treatment assignment by abstinence at baseline/study entry interaction | |||
| TES versus treatment as usual, nonabstinent at baseline/study entry (N=228) | 2.18 | 1.30, 3.68 | 0.003 |
| TES versus treatment as usual, abstinent at baseline/study entry (N=268) | 1.17 | 0.76, 1.80 | 0.489 |
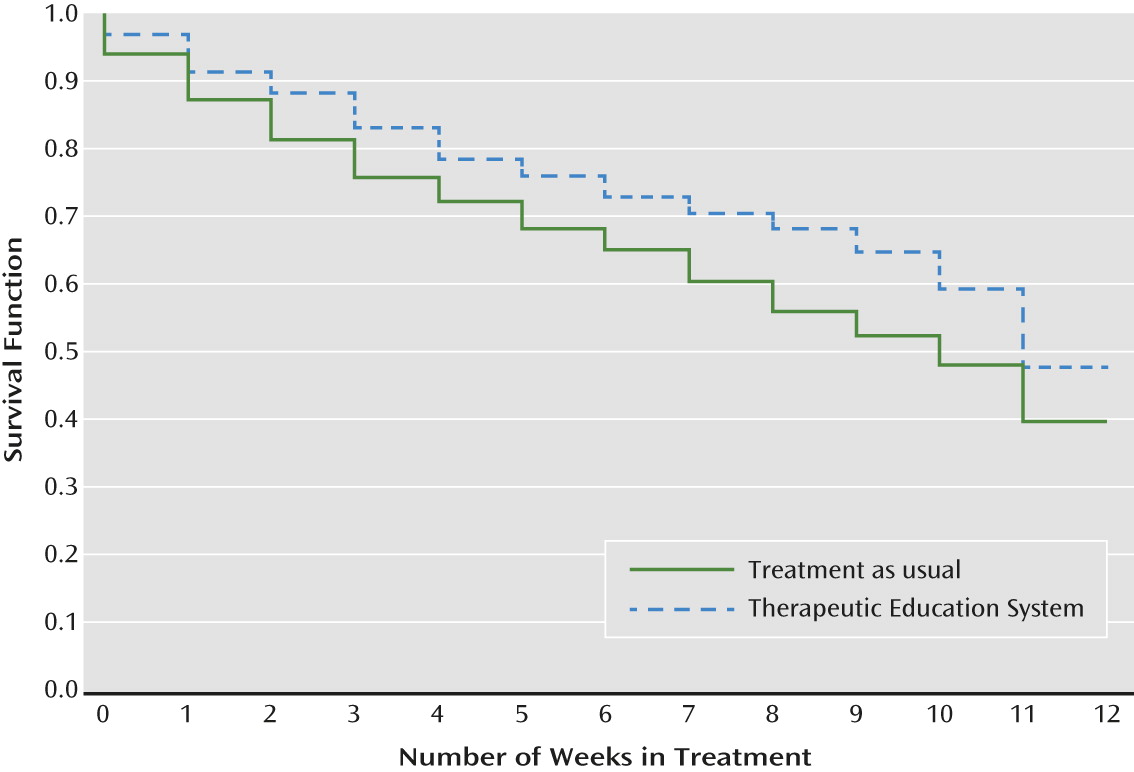
Retention in Treatment
Discussion
Strengths and Limitations
Conclusions
Acknowledgments
Footnotes
Supplementary Material
- View/Download
- 122.13 KB
References
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

