Advances in the treatment of pediatric major depressive disorder have demonstrated effective acute-phase treatments, including antidepressant medication (
1,
2), psychotherapy (
3,
4), and their combination (
5,
6). Despite these gains, 40%–70% of youths treated for depression will experience a relapse or recurrence (
7–
9). Most treatment studies focus on acute-phase outcomes, and studies that report long-term outcomes generally continue the acute-phase treatments into long-term care. Few studies have included specific sequential continuation treatment strategies developed to improve long-term outcomes.
Acute treatment strategies are designed to achieve symptom response (a significant reduction in depressive symptoms) and ultimately remission (minimal to no symptoms). Remission rates, however, have been low. Even with the combination of fluoxetine and cognitive-behavioral therapy (CBT) in the Treatment for Adolescent Depression Study (TADS), only 37% of patients remitted after 12 weeks of treatment (
10). Residual symptoms after acute treatment are common and are associated with poorer longer-term outcomes in youths (
10,
11).
Continuation treatment, on the other hand, follows acute treatment with the goals of targeting residual symptoms and preventing relapse. Over the past several years, sequential treatment models have been examined, typically beginning with pharmacotherapy for acute treatment, followed by another treatment modality to address residual symptoms once response has been achieved (
12,
13). A meta-analysis of psychotherapy delivered after pharmacotherapy suggests that sequencing these two treatment modalities may be an optimal strategy for preventing relapse (
13).
Research on continuation treatment in youths with major depressive disorder is limited. Continuing antidepressants for 6–9 months reduces relapse rates; however, some 34%–40% of these youths will relapse even while continuing on medication (
7,
14). Only three continuation studies of CBT in youths have been reported to date, with mixed results (
15–
17). In a pilot study of 46 adolescents we reported in 2008 (
17), a sequential treatment strategy using 12 weeks of fluoxetine to achieve response, followed by a specific relapse-prevention CBT delivered over 6 months, resulted in a lower risk of relapse in those who received continuation medication and relapse-prevention CBT compared with those who remained on medication alone. That study led to the larger randomized controlled trial we report here, designed to evaluate the efficacy of continuation-phase relapse-prevention CBT in reducing relapse rates. In this study, our aims were expanded to include not only relapse prevention but also shorter time to remission.
Method
This was a National Institute of Mental Health-funded single-site, randomized continuation trial in youths with major depressive disorder. The study was approved by the University of Texas Southwestern Medical Center Institutional Review Board. All participants and their parents provided written consent and assent after the purpose, procedures, and risks and benefits, as well as the rights of study subjects, were explained and all questions were answered.
Study Participants
To be included in the study, participants had to be outpatients 8–17 years of age in a general pediatric psychiatry outpatient clinic. They had to have a primary diagnosis of major depressive disorder for at least 4 weeks, with a score ≥40 on the Children’s Depression Rating Scale–Revised (CDRS-R) (
18) and a severity score ≥4 on the Clinical Global Impressions Scale (CGI-S) (
19). While major depressive disorder was the primary diagnosis, other concurrent disorders were allowed. Participants also had to be in good general medical health and of normal intelligence, and they and at least one parent had to be English speaking.
Exclusion criteria were lifetime psychotic disorder (including psychotic depression), bipolar I or II disorder, anorexia nervosa, or bulimia nervosa; alcohol or substance abuse or dependence within the past 6 months; a concurrent medical condition that would interfere with the conduct of the study or endanger the patient; concurrent use of medications with psychotropic effects; a first-degree relative with bipolar I disorder; severe suicidal ideation requiring more intensive treatment; and previous failure of an adequate trial of fluoxetine (≥4 weeks of at least 40 mg/day) or intolerance to fluoxetine. Female patients who were pregnant or lactating or were sexually active and not using adequate contraception were also excluded.
Diagnostic Evaluation
Trained master’s-level independent evaluators interviewed participants and parents, using the Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime Version (K-SADS-PL) (
20) to determine whether patients met the inclusion diagnostic criteria. Evaluators also obtained suicide and family history using the Columbia Suicide History Form and the Family History Screen. The CDRS-R, CGI-S, and Suicide Severity Rating Scale–Short were also administered.
Information regarding the course of illness and symptom severity were obtained by a child psychiatrist 1 week later through a review of the K-SADS-PL and interview of the participant and parents using the CDRS-R, the CGI-S, and the Suicide Severity Rating Scale–Short. Weekly meetings were held to review each participant for eligibility and appropriateness for the study.
Acute-Phase Treatment
After assessment, eligible participants began a 6-week course of fluoxetine. The decision to limit the acute-phase treatment to 6 weeks was based on previous trial experiences, which suggested that shortening time to remission leads to better long-term outcomes. Participants received an initial dosage of 10 mg/day of fluoxetine at the baseline visit, increasing to 20 mg/day at week 1 unless side effects were not adequately tolerated. The dosage could be increased (based on clinical judgment) to 30–40 mg/day after 4 weeks or reduced to 10 mg/day if necessary.
Medication management visits, which included assessment of symptoms and adverse events, were conducted weekly by a child psychiatrist during acute-phase treatment (weeks 0–6). Standard clinical management was provided, which followed the medication management manual from TADS (
21) and included supportive care but no specific psychotherapy. Participants who were nonadherent to medication (defined as having taken <70% of prescribed pills on two consecutive visits, based on pill count) and those who required additional treatment were discontinued.
Randomization
After 6 weeks of treatment with fluoxetine, participants were assessed by independent evaluators reassessed for response, which was defined as a decrease of at least 50% in total score on the CDRS-R. Patients who did not respond were discontinued and given appropriate referrals. Those who responded were eligible for random assignment to continuation treatment. Patients were stratified by age group (≤11 years [children] and ≥12 years [adolescents]) and remission status (CDRS-R score ≤28 or >28), and randomly assigned in a 1:1 ratio either to continue on fluoxetine only (the medication management group) or to continue on fluoxetine while also receiving relapse-prevention CBT (the medication management plus CBT group).
Continuation Treatment
Medication management.
All patients who entered the continuation phase continued to receive fluoxetine for 6 months. Medication visit schedules for both the medication management and medication management plus CBT groups were every other week for 3 months and then monthly for the remainder of continuation treatment (at weeks 20, 24, and 30). Visits lasted 30–45 minutes and consisted of assessment of symptoms and safety. Dosage was flexible during this phase and based on clinical need. Patients who needed additional interventions were discontinued from study treatment but continued with assessments.
Relapse-prevention CBT.
Patients assigned to receive medication management plus CBT began CBT sessions at week 7. A total of eight to 11 CBT sessions were planned (
Table 1). CBT visits were weekly for the first month, every other week for 2 months, and every 4–6 weeks for the last 3 months; however, the number and frequency of sessions were individualized based on patient need. Sessions typically lasted 1 hour, although the first two sessions, which included a family component, lasted 1.5 hours. Therapists were at the master’s level and above and had experience in manualized CBT. Therapy sessions were audiotaped, and a sample of sessions was reviewed by the CBT supervisors (B.D.K. and J.M.J.). Therapists also met weekly for supervision. Therapists and psychiatrists collaborated and communicated about the patient’s care.
The goals of relapse-prevention CBT include reducing residual symptoms, increasing periods of psychological well-being, and preventing relapse. Relapse-prevention CBT was developed as a brief individual treatment with a family component (an average of three sessions with the youth and one or both parents) (
17). The treatment format is modular, designed to be tailored to the specific needs of the child. The treatment also addresses risk factors for relapse (negative attributional style, family conflict, and expressed emotion) and adds elements from well-being therapy, which was adapted from Ryff and Singer’s approach (
22) and has been found to be effective in reducing relapse (
23) and promoting wellness.
Outcome Measures
Outcomes were based on blind assessments by independent evaluators at the end of acute-phase treatment (at week 6) and every 6 weeks during continuation treatment (at weeks 6, 12, 18, 24, and 30). Assessments included measures of symptoms and functioning (CGI, CDRS-R, Suicide Severity Rating Scale–Short) and course of illness using the Adolescent Longitudinal Interval Follow-Up Evaluation (adapted from the Longitudinal Interval Follow-Up Evaluation [
24]). Patients continued with assessments even if they discontinued study treatments.
The primary outcome measures for the study were time to remission and rate of relapse. Remission was defined as a CDRS-R score ≤28, with timing of remission determined through clinical interview and the Adolescent Longitudinal Interval Follow-Up Evaluation. Relapse was defined as a CDRS-R score ≥40 for at least 2 weeks, or a score <40 with significant clinical deterioration that would suggest full relapse if the patient’s treatment were not altered (
7). Only youths who had achieved remission (at any point in the study) were assessed for relapse. Time to relapse was also measured.
“Time spent well in the study” was measured with the Adolescent Longitudinal Interval Follow-Up Evaluation. Although this instrument is generally used at 6-month intervals to show status change in psychiatric disorders, in this study the independent evaluators completed the ratings at each assessment. Time spent well was defined as each week that the youth received a rating of 1 or 2 (no or minimal disorder) on the Adolescent Longitudinal Interval Follow-Up Evaluation, and it was operationalized as number of weeks with a rating of 1 or 2 divided by total number of weeks in the study.
Safety
Adverse events were assessed by the psychiatrist at each visit. Adverse events were defined as any emergent or worsening untoward event requiring intervention. Serious adverse events were defined according to U.S. Food and Drug Administration criteria.
Statistical Analysis
Demographic and clinical characteristics were compared between the medication management and medication management plus CBT groups using an independent sample t test for continuous variables and chi-square or Fisher’s exact test for categorical variables.
A Cox proportional hazards regression with adjustment for CDRS-R score, age group (child versus adolescent), and gender was used to compare time to remission and, in a separate model, time to relapse between participants in the two treatment groups. Hazard ratios were estimated and interpreted as the effect size estimator for our Cox regression. As part of the survival analysis, right censoring was used and occurred when the information available about a given participant’s survival time was incomplete because the participant did not have an event during the study. Censoring (or a censored observation) occurred when a participant dropped out or completed the study without remitting or relapsing. We also estimated the probability of remitting or relapsing, as well as the rates of remission or relapse, at each week across the 30-week continuation treatment trial.
A Poisson regression with adjustment for CDRS-R score, age group (child versus adolescent), and gender was used to compare the log “time spent well” rates, or incidence of time spent well in the study, between the two groups. Maximum likelihood estimation and type 3 tests of fixed effects were used with the Wald chi-square statistic. The Poisson model also accounted for overdispersion by adjusting the standard errors and test statistics using the Pearson scaling parameter.
Statistical analyses were performed using SAS, version 9.3 (SAS Institute, Cary, N.C.) and MedCalc for Windows, version 12.7.5 (MedCalc Software, Ostend, Belgium). All analyses were intent-to-treat. For all tests, alpha was set at 0.05 (two-tailed); to address multiple testing (multiplicity), where applicable, p values were adjusted using the false-discovery-rate procedure.
Hedges’ g was calculated to estimate effect sizes for between-group comparisons. Effect size for Hedges’ g can be interpreted using Cohen’s convention (
25), where 0.20, 0.50, and 0.80 are small, medium, and large effects, respectively.
Results
Participant Characteristics
A sample of 200 youths (54.5% female and 52% minorities) entered acute-phase treatment (the CONSORT flow diagram is available in the
data supplement that accompanies the online edition of this article). The mean age was 13.8 years (SD=2.6), and 79% were adolescents (≥12 years). The baseline CDRS-R score was 58.3 (SD=7.7).
Of the 200 patients, 144 achieved response during acute-phase treatment and were randomly assigned to continuation treatment with medication management (N=69) or with medication management plus CBT (N=75) for 6 months (
Table 2). The two treatment groups did not differ in baseline demographic and clinical characteristics, and there were no differences between those who entered continuation treatment and those who did not (N=56).
Attrition
Of the 144 youths who entered continuation treatment, 114 (79.2%) remained in the study assessments for all 30 weeks, with similar rates for the medication management plus CBT and medication management only groups (82.7% and 75.4%, respectively). The overall rate of treatment completion (defined as remaining in the assigned treatment condition up to week 30) was 66% (N=95), with a nonsignificantly higher treatment completion rate for the medication management plus CBT group than for the medication management only group (70.7% and 60.9%, respectively). The overall mean treatment exit week was similar between the two groups (26.3 [SD=6.7] and 25.2 [SD=7.1], respectively).
Fluoxetine Dosage
The number of medication management visits ranged from six to 16, with similar visit frequency between the medication management plus CBT and medication management only groups (mean=13.2 [SD=2.6] and mean=13.3 [SD=2.4], respectively). Patients in the medication management plus CBT group received an average of 9.8 CBT sessions (SD=4.0; range=0–20).
The daily dose of fluoxetine at the end of acute-phase treatment was similar for the medication management plus CBT and medication management only groups (mean=26.7 mg [SD=7.9] and mean=28.5 mg [SD=7.8], respectively). The dosage after the start of continuation treatment was based on the highest dosage received. The mean maximum daily dose received across all youths who entered continuation treatment was 32.5 mg (SD=8.5), with the medication management group receiving a significantly higher maximum daily dose than the medication management plus CBT group (mean=34.2 mg [SD=8.3] compared with mean=30.9 mg [SD=8.4]; p=0.02).
Hazard of Remission
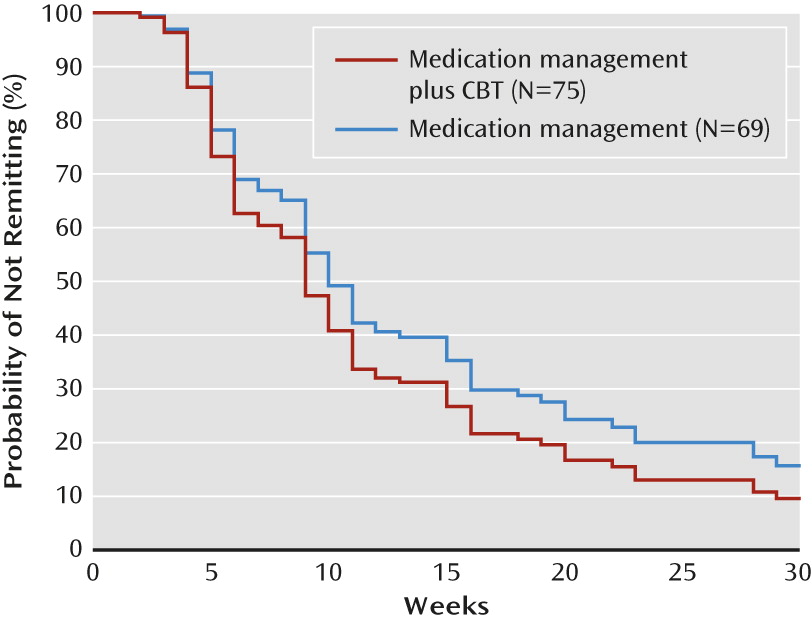
After adjustment for baseline CDRS-R score, age group, and gender, the Cox regression revealed that participants in the medication management plus CBT group did not differ significantly in time to remission from those in the medication management only group during continuation treatment (hazard ratio=1.26, 95% CI=0.87, 1.82; n.s.).
The mean time to remission for the medication management plus CBT group was 11.33 weeks (SE=0.95), compared with 13.67 weeks (SE=1.17) for the medication management only group. A log-rank test showed that the mean time to remission between the two treatment groups was not significant.
Figure 1 displays the survival curves, plotted at the mean of all covariates in the model. At weeks 12, 18, 24, and 30, the estimated probability of remitting for the medication management plus CBT group was about 68%, 79%, 86%, and 90%, respectively, compared with about 59%, 71%, 80%, and 84%, respectively, for the medication management only group. No significant treatment group effects on remission rates emerged at any individual week.
Hazard of Relapse
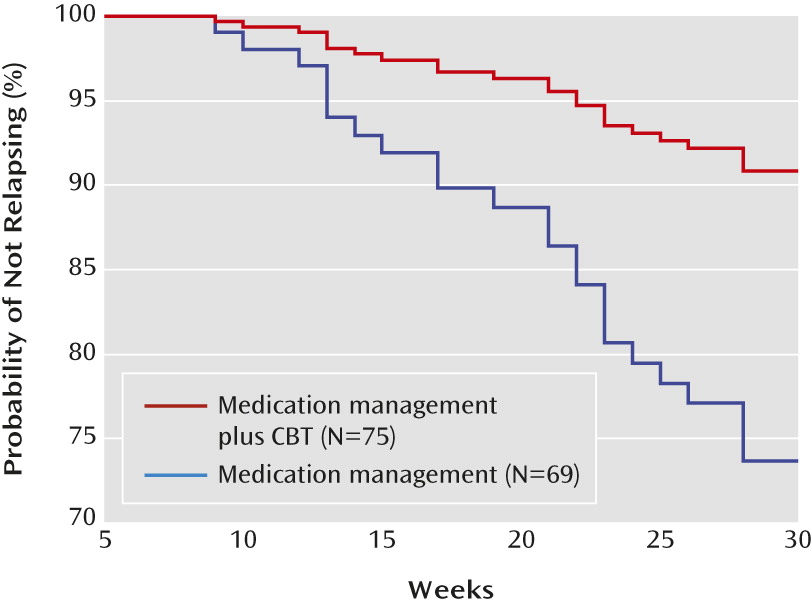
Relapse was assessed in participants who achieved remission at any time during the study (N=115). After adjustment for CDRS-R score at the end of acute-phase treatment (at 6 weeks), age group, and gender, the Cox regression revealed that participants in the medication management plus CBT group had a significantly lower risk of relapse than those in the medication management only group during the 30-week continuation phase (hazard ratio=0.313, 95% CI=0.130, 0.753; χ2=6.64, p=0.010). Thus, the hazard of relapse for those who received medication management plus CBT was 0.313 times that of those who received medication management alone.
The survival curves in
Figure 2, plotted at the mean of all covariates in the model, indicate that the survival probability of relapsing was greater at all times for medication management compared with medication management plus CBT. At weeks 12, 18, 24, and 30, the estimated probability of relapse for the medication management plus CBT group was about 1%, 3.5%, 7%, and 9%, respectively, compared with about 3%, 10%, 20.5%, and 26.5%, respectively, for the medication management only group. The two treatment groups differed significantly on relapse rates at week 24 (raw p=0.028; false-discovery-rate-adjusted p=0.049) and week 30 (raw p=0.011; false-discovery-rate-adjusted p=0.043), but not at week 12 and week 18.
During continuation treatment, the mean time to relapse for medication management plus CBT was 28.77 weeks (SE=0.48), compared with 27.13 weeks (SE=0.68) for medication management only (log-rank test: p=0.009, g=0.439). While not all analyses were replicated to examine full relapse (CDRS-R score ≥40) versus clinical deterioration, it is important to note that nine of the 17 patients in the medication management plus CBT group who relapsed (52.9%) experienced full relapse, and 15 of the 28 patients in the medication management only group who relapsed (53.6%) experienced full relapse.
Time Spent Well
The incidence of time spent well was evaluated using a Poisson regression with adjustment for CDRS-R score at the end of the acute phase, age group, and gender. Participants in the medication management plus CBT group had a significantly higher estimated rate of time spent well than those in the medication management only group (55.9% compared with 46.2%; χ2=5.48, p=0.02, g=0.395). The medication management plus CBT group spent 16.0 weeks well (SD=9.1), compared with 12.8 weeks (SD=9.5) for the medication management only group (p=0.041, g=0.342).
Safety Outcomes
The rates of adverse events leading to treatment discontinuation did not differ significantly between the two treatment groups, with 2.7% (2/75) in the medication management plus CBT group and 4.3% (3/69) in the medication management only group. Adverse events related to hypomania occurred only during acute-phase treatment, with two patients discontinuing because of hypomania (N=1) or agitation (N=1). This rate (2%) is similar to that seen in the two randomized controlled trials of fluoxetine, in which 3.1% of fluoxetine patients had mania, hypomania, or agitation (
1).
Sixteen participants experienced a total of 18 serious adverse events during continuation treatment. Seven events occurred in the medication management only group: one hospitalization for a suicide attempt, three for suicidal ideation, one for agitation, and two for medical conditions. Eleven events occurred in nine patients in the medication management plus CBT group: five hospitalizations for suicidal ideation, one case of suicidal behavior that did not result in hospitalization, and five hospitalizations for preexisting medical conditions. These events were reported to the institutional review board and the data safety monitoring board.
Discussion
To our knowledge, this is the first randomized controlled trial of a sequential treatment strategy to prevent relapse in youths with major depressive disorder. The results demonstrate that, as in adults (
13), sequencing treatments can reduce risk of relapse and lengthen time to relapse in depressed youths. Adding CBT as part of a sequential treatment strategy after response to antidepressant medication, as opposed to continuation treatment with pharmacotherapy alone, significantly reduced the hazard of relapse and lengthened time to relapse in this trial.
While the treatment was developed to prevent relapse, we anticipated that it might also shorten time to remission and therefore included this as a primary outcome measure. Our results did not support this hypothesis, however. Nonetheless, it is worth noting that those who received medication management plus CBT reached remission about 2.5 weeks earlier on average than those who were on medication alone. While not statistically significant, in the life of a child, two and a half weeks has potential clinical relevance.
An unexpected but important finding was in differences in fluoxetine dosing. Youths who received CBT had lower medication dosages yet had better outcomes than those on higher dosages in the medication management only group. The difference in dosage, while statistically significant, may not be clinically significant, but it suggests that differences in outcomes were not attributable to higher dosing in one group over the other.
The optimal timing of adding CBT is still not well understood. The guidelines from the U.K. National Institute for Health and Care Excellence indicate that youths with depression should be treated with CBT as a first-line treatment; however, acute-phase CBT, at least in more severely depressed patients, does not compare as favorably to antidepressant medication or combination treatments (
5,
26). In TADS, remission rates with CBT were not comparable to those with medication until 18–24 weeks into treatment (
11). In the present study, we did not examine the effectiveness of antidepressant medication compared with CBT; rather, we assessed whether adding CBT after initial improvement with an antidepressant would lead to greater improvement and prevent relapse. Thus, this study demonstrates that CBT can be effective as a continuation strategy. While not tested in this study, it will be important in the future to determine the optimal timing of the onset of added CBT in continuation treatment. Also worthy of study is whether a different treatment modality could be used for the acute phase.
We acknowledge that this study has some limitations. The time period for the study’s primary outcomes was 30 weeks (follow-up data were collected at weeks 52 and 78 and will be included in a subsequent report). Truncating the study outcomes at 30 weeks may have limited our ability to find significance on remission outcomes. The TADS study demonstrated that remission rates increase over time across groups (
11). In addition, while relapse rates were statistically and clinically significant (a relapse rate of 9% in the medication management plus CBT group compared with almost 27% in the medication management only group), mean time to relapse differed by only 1.6 weeks between the two groups.
Another limitation was that participants were not blind to treatment assignment, and there was a difference in clinical contact time between the two groups. We made every effort to limit additional clinic visits for the CBT condition, and in the treatment protocol we scheduled only two additional visits for these patients to reduce participant burden. Thus, all other CBT visits occurred in conjunction with medication management visits. As stated earlier, the number of medication visits was similar between the two groups. While we could have added a sham therapy to control for contact time, we wanted the study to be ecologically valid and to mirror real-world practice. We also note that results (not reported) from separate intercept-only frailty models (random-effects survival models), with therapist included as a random effect, suggest that the therapists did not introduce any significant concomitant variability in our basic findings.
Sequencing effective monotherapies improved outcomes in youths with depression in our study, and it may be a means of optimizing both medication and CBT treatment effects. This strategy is efficient, as it reduces the traditional number of CBT sessions required for positive outcomes (12–16 sessions) (
3) and thus reduces the burdens of time and cost. Furthermore, we hypothesize—although this has yet to be empirically validated—that a child who has had a positive response to medication is more fully able to participate in CBT and may be more likely to assimilate the skills and complete the practice exercises between sessions. It might be asked whether the acute-phase treatment must be medication treatment. Would the results be similar if the acute-phase treatment were CBT followed by a continuation medication treatment? In adults with major depressive disorder, such sequencing (medication following acute cognitive therapy) has not been found to result in lower relapse rates compared with continuation CBT only (
27). In addition, the CBT in this trial emphasized reducing residual symptoms, preventing relapse, and promoting wellness. Thus, as has been shown in adults (
28), continuation strategies that focus on well-being may protect youths against relapse in depression.
Continuation-phase strategies designed to reduce the high rates of relapse in depressed youths have important public health implications, as recurrence of depression is more likely in youths with multiple episodes (
9,
26). It is important that we move beyond the acute goal of getting children well to the enduring goal of promoting and maintaining wellness.