Discussion
Out of 96 consecutive patients with postpartum psychosis screened for CNS surface antibodies, four patients showed neuronal cell surface antibodies suggestive for encephalitis (4%). Two of these patients were identified as having anti-NMDA receptor encephalitis, while for the other two patients the antigen remains unknown. Notably, none of the 64 healthy postpartum comparison subjects had confirmed neuronal surface antibodies.
Two patients (2%) out of the total cohort of 96 were identified as anti-NMDA receptor antibody positive. Notably, anti-NMDA receptor encephalitis was not initially considered during the acute clinical presentation because of the absence of classical features of autoimmune encephalitis, including seizures, decreased consciousness, dyskinesia, and autonomic instability. Importantly, however, and only in retrospect, it is notable that both of the patients with anti-NMDA receptor antibody positivity had extrapyramidal symptoms with only a low dose of haloperidol, a clinical sign frequently seen among patients with autoimmune encephalitis (personal observations by M.J.T. and J.D.).
Both patients recovered after treatment with lithium, and remission was sustained, despite the absence of any steroid or immunosuppressive treatment. In general, immunotherapy and removal of an identified ovarian teratoma are the definitive treatments for anti-NMDA receptor encephalitis, both during the acute phase and for relapse prevention (
24). The effectiveness of this treatment strategy has been described in the postpartum period (
25–
27). In contrast to previous case reports of postpartum anti-NMDA receptor encephalitis, our patients displayed only a few of the classical neurological symptoms. A partial, or attenuated, anti-NMDA receptor encephalitis syndrome with predominantly psychiatric symptoms and few, if any, classical neurological features of anti-NMDA receptor encephalitis has been described in approximately 4% of all patients with NMDA encephalitis (
28,
29). Moreover, some of these patients might have antibodies with a distinct affinity and/or antigen location within the NMDA receptor complex (
13,
30). Lastly, the dynamic immunological changes of the postpartum period could also provide an explanation for the full recovery of both patients without immunotherapy in the present study.
Two patients (N=2/96, 2%) showed neuronal cell surface antibodies suggestive for encephalitis, but the corresponding antigen has remained unknown after comprehensive screening with cell-based assays. Levels of antibodies were insufficient to identify the antigen. Our hope is that with ongoing screening of additional cases, we might find additional patients with similar staining patterns and provide an opportunity for more sophisticated antigen identification approaches. In contrast to patients with anti-NMDA receptor encephalitis for whom the estimated relapse risk is 15%, the relapse risks for these two patients with unknown antigens remains unknown. We intend to closely monitor all four patients identified with cell surface antibody-associated CNS disorder and have advised them to contact us immediately if they experience any remarkable neurological or psychiatric symptoms.
A major caveat in CNS autoantibody testing has been false positivity, as previously shown in patients with a presumed diagnosis of schizophrenia who tested positive for NMDA receptor antibodies using only cell-based assay screening (
31). Among healthy cohorts, anti-NMDA receptor antibodies can often be detected in serum using cell-based assays, although IgG antibodies are rarely present (0.4%) (
32). Therefore, we previously recommended that detection of serum antibodies to neuronal cell surface antigens, including anti-NMDA receptor, should involve at least two of three antigen-binding assays: 1) immunohistochemistry with rat brain sections optimized for antigen presentation, 2) live dissociated primary neuronal cultures, or 3) a recombinant cell-based assay using transfected cells expressing the antigen of interest or confirmation by CSF testing (
10,
13,
14). Accordingly, if a patient’s serum demonstrates autoantibody positivity by cell-based assay, then at least one additional assay should be performed to verify the result. Using this confirmatory method, no false positives have yet been identified by our group, together including over 10,000 samples (unpublished observations). In addition, this method of confirmatory screening has also been shown to provide significantly higher sensitivity for detecting CNS cell surface antibodies (
10,
33).
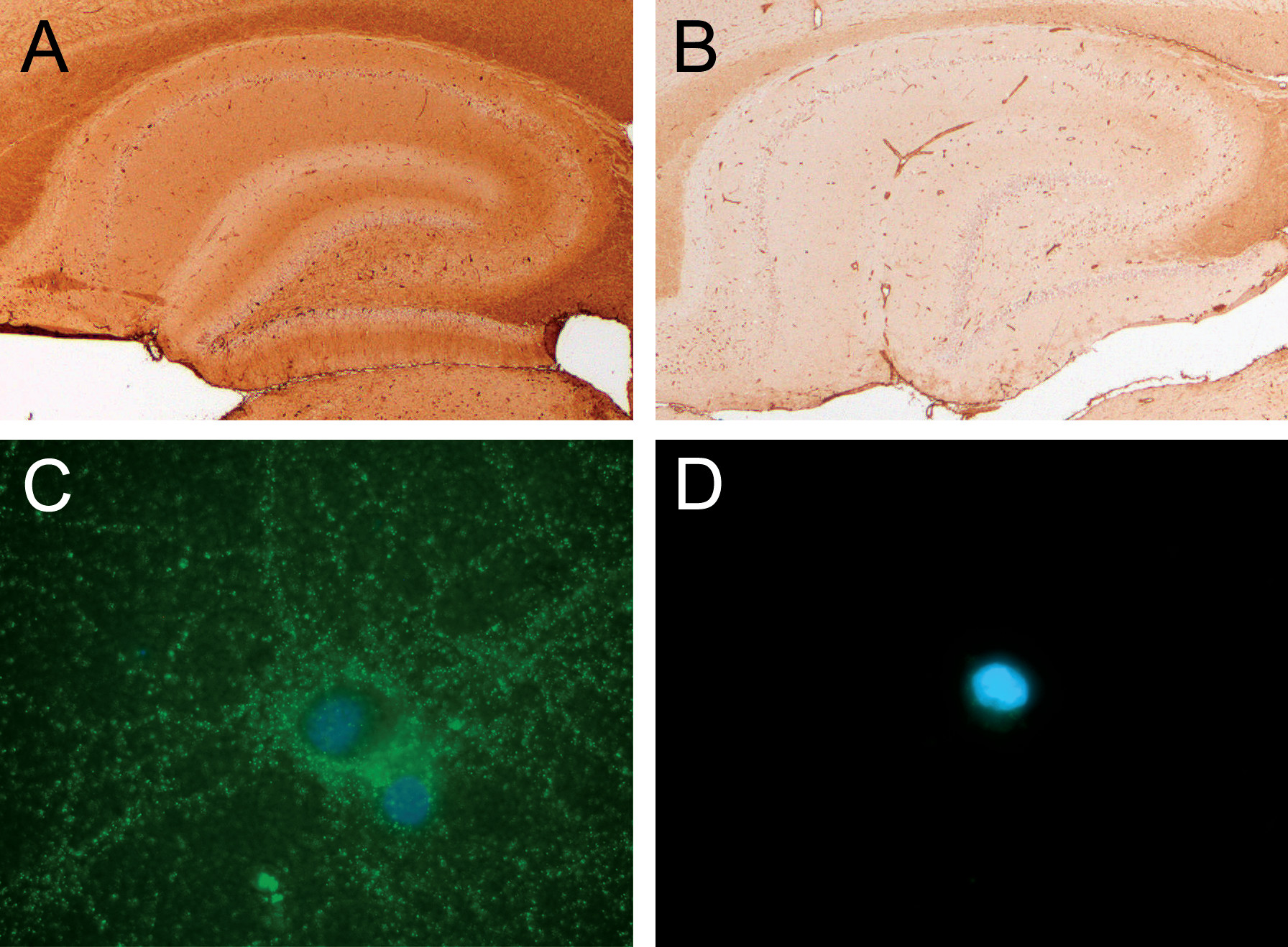
In the present study, CSF was not sampled at the time of acute symptom onset, given the lack of any overt neurological symptoms suggestive of classical anti-NMDA receptor encephalitis. CSF has been previously shown to provide a higher sensitivity and specificity for detecting CNS autoantibodies, and therefore our analysis using serum may underestimate the frequency of autoimmune encephalitis in postpartum psychosis (
14). Importantly, however, all four patients with positive immunohistochemistry labeling were confirmed using live neuron immunocytochemistry, thereby proving the presence of an antibody binding to an extracellular antigen.
To date, systematic screening for anti-NMDA receptor encephalitis in psychiatric case/control cohorts has been reported in five studies. Zandi et al. (
28) exclusively used cell-based assay and concluded that three out of 46 schizophrenia patients had GluN1 and/or GluN2 serum antibodies. Of these patients, two had decreased verbal fluency, and the remaining third had antibody titers that would fall below the currently accepted threshold for positivity (
34). Three follow-up studies did not confirm these findings using cell-based assay, among a total of 206 schizophrenia patients and 70 affective disorder patients, for which a subset of 80 subjects also tested negative by immunohistochemistry (
32,
35,
36). In the most recent study, IgG anti-GluN1 serum cell-based assay positivity was reported in eight of 1,378 schizophrenia patients (0.6%) and six of 310 affective disorder patients (1.9%); however, no additional confirmatory antibody testing or clinical follow-up was performed (
36). Taken together, the current best evidence suggests that most patients with well-established chronic psychiatric disorders are unlikely to have anti-NMDA receptor encephalitis (
24,
30).
Previous cohort studies of patients with anti-NMDA receptor encephalitis have shown that the majority of patients were women (N=468/577, 81%; median age: 21 years) (
24). Furthermore, several case reports and a larger cohort study described anti-NMDA receptor encephalitis in patients with first-episode psychosis (
29). Compared with chronic patients, the likelihood of identifying carriers of CNS autoantibodies in patients with recent-onset severe psychiatric symptomatology is expected to be higher, especially when atypical psychiatric symptoms are present (
29).
Compared with nonpuerperal psychosis, the characteristics of women with postpartum psychosis likely increases the a priori likelihood of NMDA receptor autoantibodies because these patients are female, generally young, and have first-onset episodes of atypical psychiatric symptoms, including delirium-like symptoms. In addition, following delivery, there is a well-described period of rebound immune activation, which has been widely implicated in the onset or exacerbation of multiple autoimmune disorders (
37). For example, postpartum immune activation causes clinically significant increases in thyroid peroxidase antibody levels, with elevated rates of clinical thyroid dysfunction (
38). The postpartum period is also a well-established period of substantially elevated risk for both first-onset and relapse episodes of autoimmune CNS diseases, such as multiple sclerosis (
39). Accordingly, a similar mechanism could underlie anti-NMDA receptor autoantibody pathogenesis during the postpartum period.
Patients with anti-NMDA receptor encephalitis often present with memory deficits, disorientation, and/or decreased consciousness. Furthermore, affective psychotic symptoms are relatively common. Postpartum psychosis is remarkable for having both features: an acute delirium-like appearance and prominent affective symptoms. Additionally, in the present study, both women with postpartum psychosis and anti-NMDA receptor autoantibodies exhibited extrapyramidal symptoms with low-dose haloperidol, a clinical response that is not particularly common among patients with postpartum psychosis. Interestingly, in patients with anti-NMDA receptor encephalitis, classical antipsychotics such as haloperidol seem to result in a high incidence of extrapyramidal symptoms, as well as the exacerbation of mild motor symptoms evident prior to antipsychotic treatment. Accordingly, we acknowledge the possibility that subtle neurological signs may have been unrecognized during the acute phase of the illness. Taken together, the acute onset of severe atypical psychiatric symptoms in young female patients should raise the index of suspicion for anti-NMDA receptor encephalitis, particularly when patients demonstrate neurological symptoms, including extrapyramidal side effects of low-dose antipsychotic treatment (
40). Primary screening for anti-NMDA receptor encephalitis is most optimal when performed using CSF. The problem of false positives when using serum has been recently shown in several studies (
34,
41,
42), thereby emphasizing the importance of confirming serum cell-based assay results with an independent methodology (e.g., immunohistochemistry with brain sections and/or live cultured neurons) (
33,
42).
In conclusion, we recommend NMDA receptor autoantibody screening for all patients with the acute onset of a severe psychiatric illness comorbid with neurological symptoms, including seizures, decreased consciousness, dyskinesia, or overt motor symptoms. Furthermore, anti-NMDA receptor encephalitis should be considered within the differential diagnosis for any patient with first-onset psychosis or mania, especially if multiple risk factors are present. The present study suggests that anti-NMDA receptor autoantibody screening should be considered in particular for patients with postpartum psychosis.