Most people with schizophrenia are compromised in their ability to think clearly and logically, relative to their predicted ability without the disorder or premorbid state (
1). Cognitive impairment is considered a core component of schizophrenia, affecting multiple cognitive domains, including memory, attention, and executive functioning (
2). Cognitive deficits in patients with schizophrenia predict poorer outcomes in social, occupational, and other aspects of functional outcome, ultimately affecting the ability to live and function independently (
3).
Although current therapies significantly improve the positive symptoms of schizophrenia, they do not noticeably improve cognition, and there are currently no approved pharmacological treatments for this indication. Nonpharmacological treatments have demonstrated some benefit, but they are not widely used and require considerable time and effort. For these reasons, pharmacotherapy for cognitive impairment represents a significant unmet medical need in patients with schizophrenia and is a key focus of pharmacological development by government health agencies, academic researchers, and the pharmaceutical industry.
Alpha-7 (α7) neuronal nicotinic receptors play a key role in cognitive function (
4–
6). α7 Neuronal nicotinic receptors are localized on presynaptic and postsynaptic elements in the hippocampus and cerebral cortex, regions critical to the synaptic plasticity underlying learning and memory (
6,
7). Additionally, α7 neuronal nicotinic receptors are present on the pre- and postsynapses of neurons containing other neurotransmitters (γ-aminobutyric acid, acetylcholine, glutamate) that are important for cognition (
6).
ABT-126 is a potent and highly selective partial agonist of the α7 neuronal nicotinic acetylcholine receptor. It has broad-spectrum efficacy across nonclinical models of memory consolidation, inhibitory avoidance, social recognition memory, working memory, and sensory gating deficit, which are domains of cognition that are negatively affected in schizophrenia (
8). Our previous studies in healthy volunteers, patients with schizophrenia, and patients with Alzheimer’s disease indicated that ABT-126 was generally well tolerated at all dosages tested (up to 150 mg once daily). ABT-126 displayed favorable pharmacokinetic properties, with a half-life suitable for once-daily dosing and a lack of potential drug-drug interactions. The pharmacokinetics of ABT-126 were not altered by the administration of food, and no clinically meaningful drug-drug interactions were anticipated. Most adverse events reported were mild, sporadic, and self-limiting (data on file, AbbVie). The present study was designed to assess the efficacy and safety of ABT-126 in the treatment of cognitive impairment in individuals with schizophrenia who are in the residual phase of their illness and receiving treatment with atypical antipsychotics.
Method
Study Design
This was a randomized, double-blind, placebo-controlled, parallel-group, proof-of-concept, phase 2 study. Enrolled subjects were randomized by an interactive voice response/interactive web-based system (United Biosource Corporation) in a 1:1:1 ratio to once-daily treatment with 10 mg of ABT-126, 25 mg of ABT-126, or placebo (identical-appearing capsules were used for all three groups), stratified by study site and self-reported current smoking status. Subjects and investigators remained blind to the treatment assignments throughout the study. Subjects were treated for 12 weeks at 22 centers in the United States. Eligibility was evaluated at two screening visits during the 28 to 42 days prior to randomization. The safety and efficacy of ABT-126 were evaluated throughout the study (see Table S1 in the data supplement that accompanies the online edition of this article).
The principles established by the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) consortium were followed in the execution of this study (
9,
10). Protocols, amendments, and informed consent forms were approved by local institutional review boards, and written informed consent was obtained from all subjects prior to any study procedures. An independent data monitoring committee reviewed unblinded safety data during the study.
Study Subjects
Schizophrenia patients were considered eligible if they met the following criteria: clinically stable (no hospitalization or overt destabilization in the 4 months prior to screening), age 20 to 55 years, treatment with one to two atypical antipsychotics at stable dosages, and a current diagnosis of schizophrenia confirmed by the Mini International Neuropsychiatric Interview, version 6.0 (
11). Patients had to have been diagnosed and/or treated for schizophrenia for at least 2 years. Other eligibility criteria included Positive and Negative Syndrome Scale (PANSS) item scores ≤4 for delusions, conceptual disorganization, hallucinatory behavior, and excitement and a Calgary Depression Scale for Schizophrenia total score ≤10 at screening.
Patients were excluded if they had clinically significant extrapyramidal symptoms, significant neurological disease, uncontrolled mental illness, a history of substance abuse, hepatitis B, hepatitis C, or HIV infection; and prior (>8 weeks) treatment with clozapine, tricyclic antidepressants, or monoamine oxidase inhibitors. Allowable anxiolytics and hypnotics were restricted to eszopiclone, zopiclone, zolpidem, ramelteon, trazodone, estazolam, lorazepam, oxazepam, and temazepam.
Primary Efficacy Measure
The primary efficacy measure was the change from baseline to week 12 compared with placebo on the MATRICS Consensus Cognitive Battery (MCCB) composite score. The MCCB comprises 10 tests of cognitive functioning and assesses seven domains of cognition (speed of processing, verbal learning, working memory, reasoning and problem solving, visual learning, attention/vigilance, and social cognition). The MCCB has shown good test-retest reliability, discriminates patients with schizophrenia from healthy subjects, and correlates with functional status (
12,
13). Scoring of the MCCB is based on a normative distribution (t scores) and a mean score of 50 (SD=10) in a healthy population. All raters were trained and certified for MCCB administration by NeuroCog Clinical Trials (Chapel Hill, N.C.). During the execution of the trial, each test was reviewed for scoring accuracy by two independent experts, and any scoring discrepancies were reconciled with the rater.
Secondary Efficacy Measures
The secondary efficacy measures included total score on the UCSD Performance-Based Skills Assessment–2 (UPSA), total score on the 16-item Negative Symptom Assessment, and the seven domain scores of the MCCB.
The UPSA is a role-play test designed to evaluate cognitive functional capacity in six selected domains of basic living skills in individuals with schizophrenia: organization/planning, financial skills, communication, transportation, household management, and medication management. Subjects being tested utilize props to demonstrate how they perform everyday activities and are assessed on their actual performance. Scores range from 0 to 20 for each subtest and 0 to 120 for the total score (
14). The UPSA has established reliability and validity, and it is significantly correlated with the MCCB (
15). In this study, experts at NeuroCog Clinical Trials trained raters, evaluated the scoring of each test, and reconciled any discrepancies with the rater.
The total score on the Negative Symptom Assessment is based on the 16 items of the instrument plus a one-item global rating designed to measure the severity of specific negative symptoms in schizophrenia (
16). Validation studies have demonstrated greater sensitivity compared with other negative symptom scales in assessing change in negative symptom severity (
17). Factor analysis indicates five negative symptom factors; communication, affect, social activity, motivation, and motor retardation. Items are rated on a 6-point scale that ranges in severity from 1 (absence of negative symptom or normal responsiveness) to 6 (extreme evidence of negative symptom).
Clinical symptoms were assessed throughout the study using the PANSS and the Clinical Global Impressions severity score (CGI-S). The Cambridge Neuropsychological Test Automated Battery (CANTAB) for schizophrenia was also included as a secondary cognitive battery. A single measure from each of the CANTAB tests was identified as being primary based on clinical relevance. Efficacy measures were assessed throughout the study (see Table S1 in the data supplement). Smoking status was obtained by self-report, and smoking was permitted ad libitum throughout the study, including before or between individual tests during cognitive testing.
Safety, Tolerability, and Pharmacokinetics
The safety and tolerability of ABT-126 were assessed with adverse event reports, physical examinations, clinical laboratory tests, ECG, and vital sign measurements. Suicidal ideation and behaviors were assessed with the Columbia-Suicide Severity Rating Scale. Treatment-emergent adverse events were ascertained at each visit as well as by spontaneous report. Adverse events were coded according to the Medical Dictionary for Regulatory Activities (MedDRA), version 14.0, and tabulated by preferred term. Study investigators rated the severity of each adverse event (mild, moderate, or severe) and the relationship of the adverse event to the use of the study drug (probably related, possibly related, probably not related, or not related).
Pharmacokinetic samples were collected at weeks 1, 2, 4, 6, 8, and 12 (or at premature discontinuation) (one sample per visit, six samples planned per subject). ABT-126 plasma concentrations were determined by the sponsor using a validated liquid chromatography method with tandem mass spectrometric detection. Study drug adherence was also monitored throughout the study.
Statistical Analysis
A sample size of 63 subjects per treatment group was chosen to provide 80% power to detect an effect size of 0.45 for a one-sided test at an alpha of 0.050. All efficacy analyses were conducted on the intent-to-treat data set (N=203). Data collected more than 3 days after the last dose of the study drug were not included in efficacy analyses, except in one sensitivity analysis for the MCCB composite score. Unless otherwise specified, for all efficacy analyses, “baseline” referred to the last nonmissing observation prior to the first dose of study drug, and “final assessment” referred to the last nonmissing observation after the first dose of study drug and no more than 3 days after the last dose of study drug.
The primary efficacy analysis of the MCCB composite score was performed using a likelihood-based, mixed-effects repeated measures analysis of the change from baseline to weeks 6 and 12. The model included fixed categorical effects for treatment, site, visit, and treatment-by-visit interaction, with continuous fixed covariates for baseline score and the baseline score-by-visit interaction. Pairwise comparisons between each ABT-126 dosage group and placebo were performed with one-sided tests at an alpha of 0.050. The one-sided test was selected because ABT-126 had to demonstrate improvement compared with placebo to be considered effective. The two-sided test was performed post hoc, and results are reported. The secondary efficacy analysis was performed using the analysis of covariance (ANCOVA) model with factors of treatment, site, and baseline score as a covariate on change from baseline to final assessment.
Prespecified subgroup analyses were performed on change from baseline to final MCCB composite and domain scores for gender, age, duration of illness, cigarette smoking status, and baseline level of severity on the MCCB and the PANSS. These were performed using an ANCOVA model with the terms of treatment, site, subgroup variable, the treatment-by-subgroup variable interaction, and baseline score as a covariate. The homogeneity of treatment effect across strata was evaluated by testing the treatment-by-subgroup interaction term at an alpha of 0.100.
All safety analyses were performed on all subjects who took at least one dose of the study drug (N=203). Fisher’s exact test was used to compare percentage of subjects with each adverse event between each ABT-126 dose and placebo. One-way analysis of variance was used to compare change from baseline to each visit between each ABT-126 dose and placebo for continuous variables. All statistical tests for safety data were two-tailed at an alpha of 0.050.
Plasma concentrations of ABT-126 for each dosage were combined across all visits. The plasma concentrations were categorized on the basis of time since administration of the previous dose of ABT-126.
Results
Subject Disposition and Baseline
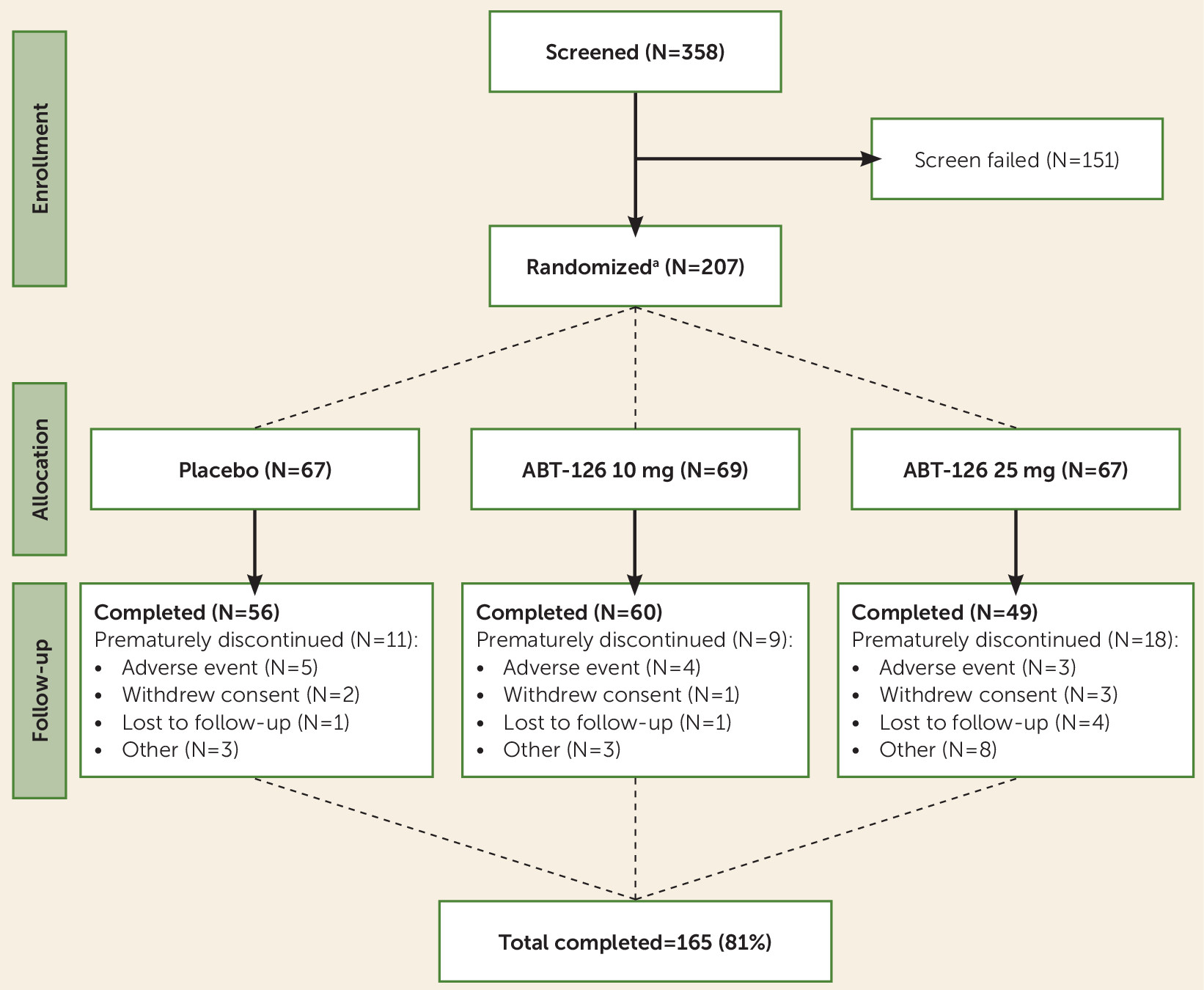
Subject disposition is shown in
Figure 1. A total of 207 subjects were randomized, 203 of whom were treated at 22 sites in the United States, and 165 (81%) completed the study. The mean age was 42 years (SD=10), and the mean time since diagnosis was 17 years (SD=10) (
Table 1). Approximately 67% (134/203) of subjects used tobacco products (
Table 1). Subjects were well balanced across treatment groups at baseline in demographic characteristics, psychiatric history, and cognitive status (
Tables 1 and
2).
Duration of Exposure and Compliance
The overall duration of exposure to the study drug ranged from 2 days to 99 days across treatment groups. There was a statistically significant difference in duration of treatment across the groups (p=0.033), with a shorter duration of exposure among subjects in the ABT-125 25 mg group (mean=68 days, SD=29) compared with the ABT-126 10 mg group (mean=78 days, SD=19) and the placebo group (mean=76 days, SD=20).
The individual ABT-126 plasma concentrations at the different visits suggested that approximately 40% of the subjects receiving ABT-126 were nonadherent (plasma concentrations <25% of the predicted mean Cmin for a given ABT-126 dose), at least occasionally; 15% to 18% were consistently nonadherent (i.e., in at least three visits), and 24% appeared to be nonadherent at the final visit. Approximately 15% of subjects had nonmeasurable ABT-126 plasma levels on at least one visit. Nonadherence rates were similar across treatment groups (ABT-125 10 mg and 25 mg) and by smoking status (data on file, AbbVie). ABT-126 plasma exposures and estimated clearance values were comparable in smokers and nonsmokers (data on file, AbbVie).
Efficacy
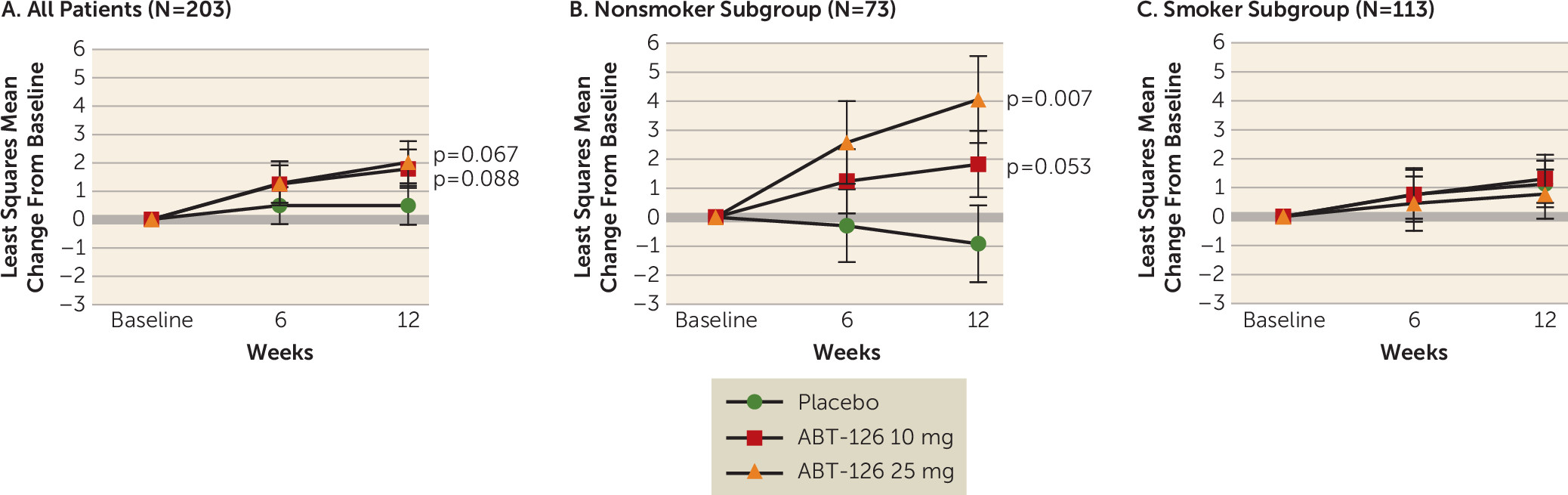
The primary efficacy analysis showed improvement in cognition relative to placebo, although it fell short of significance, based on the mean change from baseline to week 12 on the MCCB composite score in the total sample for both the ABT-126 10 mg group (least squares mean difference from placebo=+1.3, SE=1.0, p=0.088) and the ABT-126 25 mg group (least squares mean difference from placebo=+1.5, SE=1.0, p=0.067). At week 12, the least squares mean change from baseline was +1.8 (SE=0.7) for the ABT-126 10 mg group, +2.0 (SE=0.7) for the ABT-126 25 mg group, and +0.5 (SE=0.7) for the placebo group (
Figure 2A). Post hoc two-sided tests resulted in p values of 0.176 and 0.134 for the ABT-126 10 mg and 25 mg groups, respectively. Statistically significant improvements or improvements just falling short of significance were noted across a number of MCCB domains, particularly verbal learning (25 mg: least squares mean difference=2.3, SE=0.9, p=0.063), working memory (25 mg: least squares mean difference=2.1, SE=0.9, p=0.054), and attention/vigilance (10 mg: least squares mean difference=0.6, SE=1.0, p=0.040; 25 mg: least squares mean difference=0.9, SE=1.2, p=0.036).
Of all the prespecified subgroup analyses performed (gender, age, duration of illness, smoking status, baseline level of severity), only the interaction between smoking status and treatment was statistically significant (p=0.015). Nonsmokers (N=69) demonstrated differences from placebo of 2.9 for 10 mg (SE=1.4, p=0.021) and 5.2 for 25 mg (SE=1.6, p=0.001) on the MCCB composite score, and no differences were observed in smokers (N=113). Among nonsmokers in the ABT-126 25 mg group (N=19), significant improvements from placebo were observed at final visit for verbal learning (least squares mean difference=5.5, SE=1.9, p=0.003), working memory (least squares mean difference=5.4, SE=2.0, p=0.003), and attention/vigilance (least squares mean difference=8.7, SE=2.5, p<0.001).
The effect of smoking status was further explored using a post hoc mixed model for repeated measurement analysis. Within the nonsmoker subgroup, there were statistically significant improvements compared with placebo in the change from baseline to week 12 on the MCCB composite score in the ABT-126 25 mg group, while the relationship fell short of significance in the 10 mg group (25 mg: least squares mean difference=+5.0, SE=2.0, two-sided p=0.007; 10 mg: least squares mean difference=+2.7, SE=1.7, p=0.053) (
Figure 2B). In the smoker subgroup, no significant changes were noted (
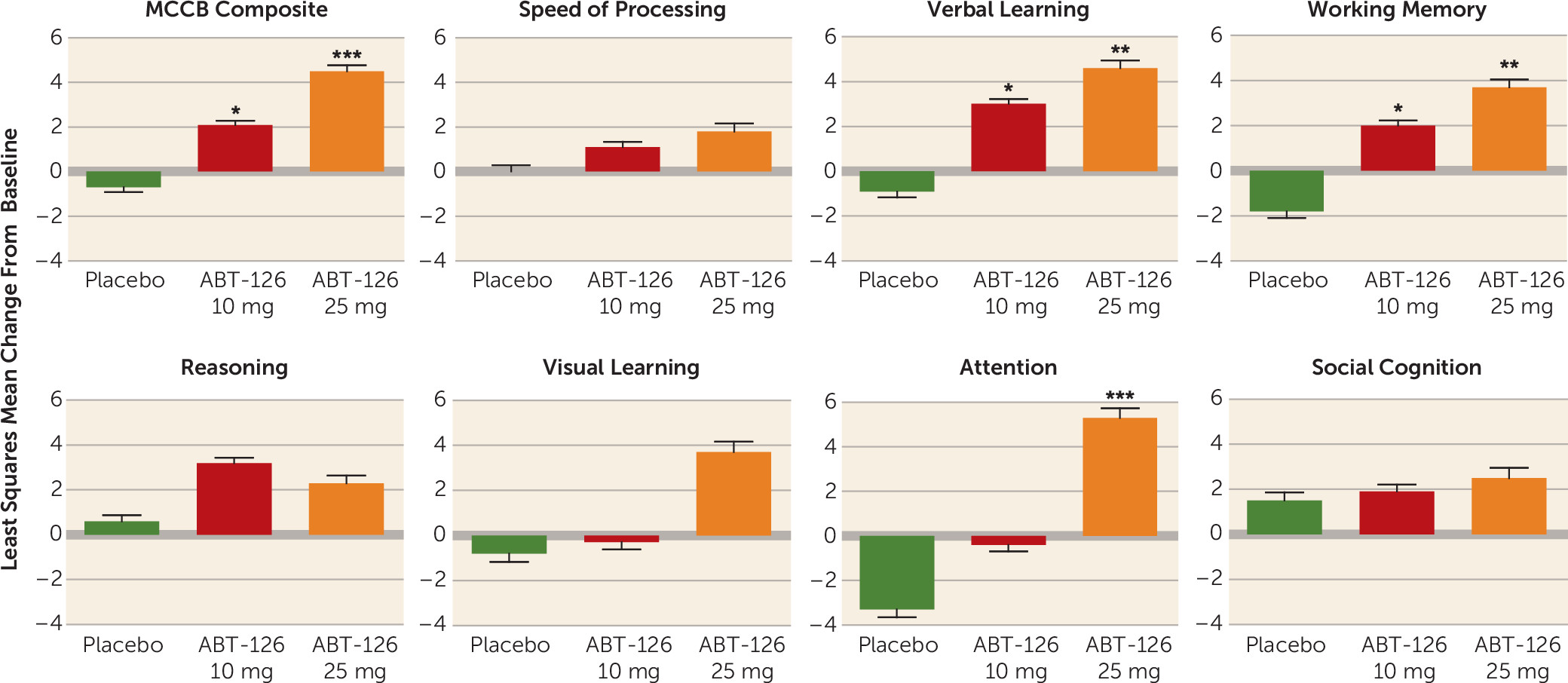
Figure 2C). The treatment effect size (Cohen’s d) for the MCCB in the nonsmoker group was 0.84 for the ABT-126 25 mg treatment group. Within the nonsmoker subgroup, there were statistically significant improvements in the ABT-126 treatment groups compared with placebo for three of the seven MCCB domains—verbal learning, working memory, and attention (
Figure 3)—whereas no significant changes were observed on any domain scores in the smoker subgroup. Additionally, the magnitude of change increased monotonically with ABT-126 dosage in six of the seven MCCB domains in the nonsmoker subgroup (
Figure 3).
There was no treatment effect on cognitive functional capacity as measured by the UPSA total score (
Table 3); however, there were dose-related numerical improvements in the subset of nonsmokers.
In the nonsmoking subgroup, significant results were seen on prespecified primary measures from two CANTAB tests. The ABT-126 25 mg group (N=19) showed statistically significant improvements at final assessment on delayed match to sample percent correct (12-second delay) compared with the placebo group (least squares mean difference=14.4, SE=8.0, p=0.037). The ABT-126 25 mg group also showed superiority on the rapid visual information processing test compared with the placebo group (least squares mean difference=0.04, SE=0.01, p=0.007).
There were no statistically significant treatment-by-smoking status interactions for the remaining secondary measures (
Table 3). The mean values on the PANSS did not change throughout the treatment period.
Safety
Fifty-four percent (74/136) of subjects treated with ABT-126 and 57% (38/67) of those treated with placebo reported an adverse event (see Table S2 on the online data supplement). Most adverse events were considered by study investigators to be mild or moderate in severity. There were no statistically significant differences between either of the ABT-126 groups and the placebo group in the incidence of adverse events overall or adverse events considered possibly or probably related to the study drug in subjects treated with ABT-126 compared with those treated with placebo. The adverse events most commonly reported in the ABT-126 groups were diarrhea, dizziness, headache, nausea, fatigue, and nasopharyngitis (see Table S2). Nine subjects (4%) in the study reported a serious adverse event (three in the placebo group and six in the ABT-126 groups) (see Table S2).
Thirteen subjects in the ABT-126 groups (6%) prematurely discontinued the study because of an adverse event—6% of the 10 mg group (N=69) and 6% of the 25 mg group (N=67)—compared with 5% of placebo group (N=67) (see Table S2). Worsening of schizophrenia symptoms (reported for two subjects in the placebo group and one in each of the ABT-126 groups) was the only adverse event that led to premature discontinuation for more than one subject. No subjects died during the study (see Table S2). There were no consistent, clinically meaningful differences in laboratory, vital sign, or ECG outcomes.
Discussion
ABT-126, a selective α7 neuronal nicotinic receptor partial agonist, demonstrated a dose-dependent improvement in cognition that trended toward but did not achieve statistical significance in a population of stable patients with schizophrenia. In prespecified subgroup analyses by smoking status, there was no effect in study subjects who were smokers, but a large effect in nonsmoking subjects. Nonsmoking subjects treated with ABT-126 had a significant monotonic dose-response on the MCCB composite score, with a Cohen’s d effect size >0.8. The MCCB results in nonsmokers were internally consistent across individual domains: changes for both dosage groups were numerically larger than those for the placebo group in every domain, with dose-dependent improvements in six of the seven MCCB domains and statistically significant differences observed in the verbal learning, working memory, and attention domains. These improvements were consistent with the changes in the CANTAB test battery domains, which were conducted at different time points throughout the study and in which significant differences were observed on attention and working memory. Although not statistically significant, the model-based Cohen’s d effect size of 0.35 observed for the UPSA in the ABT-126 25 mg group suggests the possibility that a statistically significant difference might be demonstrated with a larger sample size.
The nature of subset analyses raises questions about the credibility and reproducibility of such findings. In this case, there is internal consistency in the results for the nonsmoking subgroup (dose-response across domain scores, consistent results in similar domains from the CANTAB battery domains, and dose-related numerical improvements on the UPSA). In addition, there is scientific plausibility to these findings. Other investigators have demonstrated procognitive effects with acute nicotine treatment, as well as with chronic treatment with α7 receptor agonists, in individuals with schizophrenia (
19–
22). These improvements have generally been limited to either nonsmokers or smokers who have abstained from nicotine for a considerable period (
19,
22). Nicotine is a potent, broad-spectrum nicotinic receptor agonist. Nicotine exposure results in nicotinic receptor desensitization, which lasts from hours to days (
23,
24). The current hypothesis is that chronic nicotine exposure from tobacco smoke renders nicotinic receptors ineffective (
25). Early trials with nicotinic agonists were conducted either in subjects who did not smoke or in subjects who smoked but were required to abstain from smoking for a period of time prior to cognition testing (
22,
26,
27). Even some large-scale drug trials with nicotinic agonists conducted by other pharmaceutical companies employed smoking restrictions in their trials (
27). The results on the MCCB in a 319-subject trial with encenicline, another α7 neuronal nicotinic receptor agonist, were highly consistent with our data (
28). Although the results were not significantly different between smokers and nonsmokers, detailed subgroup analyses were not disclosed. However, restrictions on smoking prior to cognition testing were applied (see
https://clinicaltrials.gov/ct2/show/NCT00968851), and thus a lack of effect of smoking on cognition could not be concluded. In our trial, smoking subjects were permitted to smoke ad libitum, with no restrictions prior to or during cognition testing, in order to ensure that the results would be generalizable to the patient population.
No meaningful changes in the severity of schizophrenia symptoms, as measured by the PANSS and its positive and negative symptom subscales, and specifically negative symptoms, as measured by the Negative Symptom Assessment, were observed in the total sample or in subgroups defined by smoking status or baseline level of negative symptoms. The mean PANSS total score fluctuated only mildly across all groups during the treatment period, indicating that subjects remained relatively stable throughout the study. These results suggest that the improvements in cognition during treatment with ABT-126 were independent of changes in schizophrenia symptoms and that ABT-126 did not worsen the underlying psychotic illness.
The incidence of adverse events for ABT-126 was similar to that for placebo. ABT-126 did not appear to be associated with gastrointestinal effects, such as nausea and vomiting, which are common for nicotine or other nonselective nicotinic agonists (
29). Other selective α7 agonists, as well as ABT-126 in the Alzheimer’s disease population, have been associated with constipation in a dose-dependent fashion (
30), but that was not the case in this study. The rate of treatment discontinuation was higher in the 25 mg group compared with the 10 mg and placebo groups, but that is not reflective of safety or tolerability. The incidence of discontinuations due to adverse events was similar across all treatment groups. The higher rate of discontinuation in the 25 mg group was due to “other” causes. There did not appear to be evidence of activation, as the rate of schizophrenia exacerbations or worsening schizophrenia with active treatment was similar to that with placebo. Lastly, there were no notable trends on laboratory, ECG, suicidality, or physical examination assessments.
In conclusion, ABT-126 demonstrated a procognitive effect in stable, nonsmoking subjects with schizophrenia, as demonstrated by improvements on the MCCB. These data support further evaluation of ABT-126 in both smokers and nonsmokers. ABT-126 was generally safe and well tolerated in this population.