Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses
Abstract
Objective:
Method:
Results:
Conclusions:
Method
Search Strategy
Study Inclusion Criteria
Study Tabulation
Statistical Analysis
Results
| Intervention, First Author, and Year | Daily Dose | Design | Study Duration | N | Antidepressant | Primary Outcome | Resultb |
|---|---|---|---|---|---|---|---|
| S-Adenosylmethionine (SAMe) | |||||||
| Alpert (12) 2004 | 1600 mg (target dose) | Open-label; treatment-resistantc | 6 weeks | 30 | Fluoxetine/paroxetine/citalopram ≥20 mg/day, escitalopram ≥10 mg/day, sertraline ≥50 mg/day, or venlafaxine ≥75 mg/day | HAM-D 17 | + |
| Bambling (13) 2015 | 1600 vs. 800 mg | Dosage-blind; open-label; RA; treatment-resistantd | 15 weeks | 36 | Any SSRI | BDI | +e |
| De Berardis (14) 2013 | 800 mg | Open-label; treatment-resistantf; single-blind | 8 weeks | 25 | SSRI, SNRI, agomelatine, mirtazapine, or bupropion at adequate dose for at least 6 weeks | HAM-D | + |
| Papakostas (15) 2010 | 1600 mg (target dose) vs. placebo | Double-blind; RCT | 6 weeks | 73 | SSRI or SNRI at adequate and stable dose for at least 4 weeks | HAM-D 17 | n.s.(+)g |
| Folic acid | |||||||
| Coppen (16) 2000 | 0.5 mg vs. placebo | Double-blind; RCT | 10 weeks | 127 | Fluoxetine 20 mg/day | HAM-D 17 | +h |
| Resler (17) 2008 | 10 mg vs. placebo | Double-blind; RCT | 6 weeks | 27 | Fluoxetine 20 mg/day | HAM-D 17 | + |
| Basoglu (18) 2009 | 2.5 mg vs. escitalopram only | RA; open-label | 6 weeks | 42 | Escitalopram 10 mg/day | MADRS | n.s. |
| Venkatasubramanian (19) 2013 | 1.5 vs. 5 mg | Dosage-blind; open-label, RA | 6 weeks | 42 | Fluoxetine 20 mg/day | HAM-D 17 | + |
| Bedson (20) 2014 | 5 mg vs. placebo | Double-blind; RCT | 12 weeks | 475 | Any antidepressant at adequate dose and duration | BDI-II | n.s. |
| Folic acid (FA) and vitamins B12 and B6 | |||||||
| Almeida (21) 2014 | FA 2 mg + B12 0.5 mg + B6 25 mg vs. placebo | Double-blind; RCT | 52 weeks | 153 | Citalopram 20–40 mg/day | MADRS | n.s.(+)i |
| Methylfolate and folinic acid (ME: methylfolate; FO: folinic acid) | |||||||
| Godfrey (22) 1990 | ME 15 mg vs. placebo | Double-blind; RCT | 6 months | 24j | Undefined antidepressant treatment | HAM-D 17 | + |
| Alpert (23) 2002 | FO 30 mg (target dose) | Open-label; treatment-resistantc | 8 weeks | 22 | SSRI or venlafaxine for at least 4 weeks | HAM-D 17 | +k |
| Papakostas (24) 2012 | ME 15 mg (target dose) vs. placebol | Double-blind; RCT; SSRI-resistantm | Fluoxetine/paroxetine/citalopram ≥20 mg/day, escitalopram ≥10 mg/day, or sertraline ≥50 mg/day | HAM-D 17 | |||
| Trial 1 | 60 days | 148 | n.s. | ||||
| Trial 2 | 60 days | 75 | + | ||||
| Vitamin B12 | |||||||
| Syed (25) 2013 | 1000 μg i.m. vs. antidepressant only | Open-label; RA; single-blind | 6 weeks | 73 | TCA equivalent to imipramine 100–200 mg/day or SSRI equivalent to fluoxetine 20–40 mg/day | HAM-D 20 | + |
| Intervention, First Author, and Year | Daily Dose | Design | Study Duration | N | Antidepressant | Primary Outcome | Resultb |
|---|---|---|---|---|---|---|---|
| EPA/DHA | |||||||
| Su (26) 2003 | 4.4 g EPA + 2.2 g DHA vs. placebo | Double-blind; RCT | 8 weeks (plus 1 week placebo run-in) | 28 | Any antidepressantsc | HAM-D 21 | + |
| Carney (27) 2009; coronary heart disease patientsd | 0.93 g EPA + 0.75 g DHA vs. placebo | Double-blind; RCT | 10 weeks (plus 2 week placebo run-in) | 122 | Sertraline 50 mg/day | BDI-II | n.s. |
| Gertsik (28) 2012 | 1.8 g EPA + 0.4 g DHA vs. placebo | Double-blind; RCT | 8 weeks (plus 1 week placebo run-in) | 42 | Citalopram 20–40 mg/day (titrated depending on response) | HAM-D 21 | + |
| EPA vs. DHA | |||||||
| Mozaffari-Khosravi (29) 2013 | 1 g EPA vs. 1 g DHA vs. placebo | Double-blind; RCT | 12 weeks | 81 | Any antidepressant | HAM-D 17 | +e |
| Ethyl-EPA (E-EPA) | |||||||
| Nemets (30) 2002 | 2 g vs. placebo | Double-blind; RCT | 4 weeks | 20 | Any antidepressantf | HAM-D 24 | + |
| Peet (31) 2002 | 1 g vs. 2 g vs. 4 g vs. placebo | Double-blind; RCT | 12 weeks | 70 | Any antidepressant | HAM-D 17 | +g |
| Jazayeri (32) 2008 | 1 g E-EPA + fluoxetine vs. 1 g E-EPA monotherapy vs. fluoxetine only | Double-blind; RCT; double dummy | 8 weeks | 60 | Fluoxetine 20 mg/day | HAM-D 17 | +h |
| Bot (33) 2010; diabetes patientsi | 1 g vs. placebo | Double-blind; RCT | 12 weeks | 25 | Any antidepressant | MADRS | n.s. |
| Intervention, First Author, and Year | Daily Dose | Design | Study Duration | N | Antidepressant | Primary Outcome | Resultb |
|---|---|---|---|---|---|---|---|
| Tryptophan | |||||||
| Levitan (34) 2000 | 4 g (target dose) vs. placebo | Double-blind; RCT | 8 weeks (plus 5-day placebo run-in) | 39 | Fluoxetine 20 mg/day | HAM-D 29 | n.s. |
| l-Tryptophan | |||||||
| Shaw (35) 1975 | Clomipramine only vs. clomipramine + 6 g l-tryptophan vs. clomipramine + desipramine vs. desipramine + 6 g l-tryptophan | Double-blind; RCT; triple dummy | 4 weeks | 54 | Clomipramine ≤175 mg/day or desipramine ≤225 mg/day | BDI | n.s. |
| Thomson (36) 1982 | 3 g l-tryptophan only vs. amitriptyline only vs. 3 g l-tryptophan + amitriptyline vs. placebo only | Double-blind; RCT | 12 weeks (plus 1 week placebo run-in) | 115 | Amitriptyline 150 mg/day | HAM-D 18 | +c |
| dl-Tryptophan | |||||||
| Glassman (37) 1969 | 12, 15, or 18 g (depending on body weight) vs. placebo | Double-blind; CT | 3 weeks | 20 | Phenelzine 60 mg/day | HAM-D (modified) | + |
| Ayuso Gutierrez (38) 1971 | 6 g vs. placebo | Double-blind; RCT | 3 weeks | 30 | Nialamide 500 mg/day i.m. (target dose) | HAM-D | + |
| Walinder (39) 1976 | 0.1 g/kg of body weight vs. placebo | Double-blind; RCT | 3.5 weeks | 26 | Clomipramine 150 mg/day | Cronholm-Ottosson Depression Scale | + |
| Walinder (40) 1981 | 0.1 g/kg of body weight vs. placebo | Double-blind; RCT | 3 weeks | 26 | Zimelidine 200 mg/day | Cronholm-Ottosson Depression Scale | n.s. |
| l-5-HTP | |||||||
| Nardini (41) 1983 | 300 mg vs. placebo | Double-blind; RCT | 4 weeks | 26 | Chlorimipramine 50 mg/day | HAM-D | + |
| Intervention, First Author, and Year | Daily Dose | Design | Study Duration | N | Antidepressant | Primary Outcome Measure | Resultb |
|---|---|---|---|---|---|---|---|
| Zinc | |||||||
| Siwek (42) 2009 | 25 mg vs. placebo | Double-blind; RCT | 12 weeks | 60 | Imipramine 100–200 mg/day | HAM-D 17 | n.s.c |
| Ranjbar (43) 2013 | 25 mg vs. placebo | Double-blind; RCT | 12 weeks | 44 | Citalopram 20–60 mg/day or fluoxetine 20–60 mg/day | BDI | + |
| Vitamin C | |||||||
| Amr (44) 2013; pediatric patients | 1 g vs. placebo | Double-blind; RCT | 6 months | 27 | Fluoxetine 10–20 mg/day | CDRS | + |
| Sahraian (45) 2015 | 1 g vs. placebo | Double-blind; RCT | 8 weeks | 43 | Citalopram 20 mg/day | HAM-D 21 | n.s. |
| Vitamin D3 | |||||||
| Khoraminya (46) 2013 | 1500 IU vs. placebo | Double-blind; RCT | 8 weeks | 42 | Fluoxetine 20 mg/day | HAM-D 24 | + |
| Zanetidou (47) 2011; patients age >65 | 300,000 IU vs. antidepressant only | Open-label; CT | 4 weeks | 24d | Any antidepressant | HAM-D | + |
| Inositol | |||||||
| Levine (48) 1999 | 12 g vs. placebo | Double-blind; RCT | 4 weeks | 36 | SSRI | HAM-D | n.s. |
| Nemets (49) 1999 | 12 g vs. placebo | Double-blind; RCT | 4 weeks | 42 | SSRI | HAM-D 24 | n.s. |
| Amino acids | |||||||
| Ille (50) 2007 | Individualized amino acid mixture vs. placebo | Double-blind; RCT | 4 weeks | 40 | Mirtazapine (no dose specified) | HAM-D | + |
| Creatine | |||||||
| Lyoo (51) 2012 | 5 g (target dose) vs. placebo | Double-blind; RCT | 8 weeks | 52 | Escitalopram 20 mg/day | HAM-D 17 | + |
One-Carbon Cycle Nutraceuticals
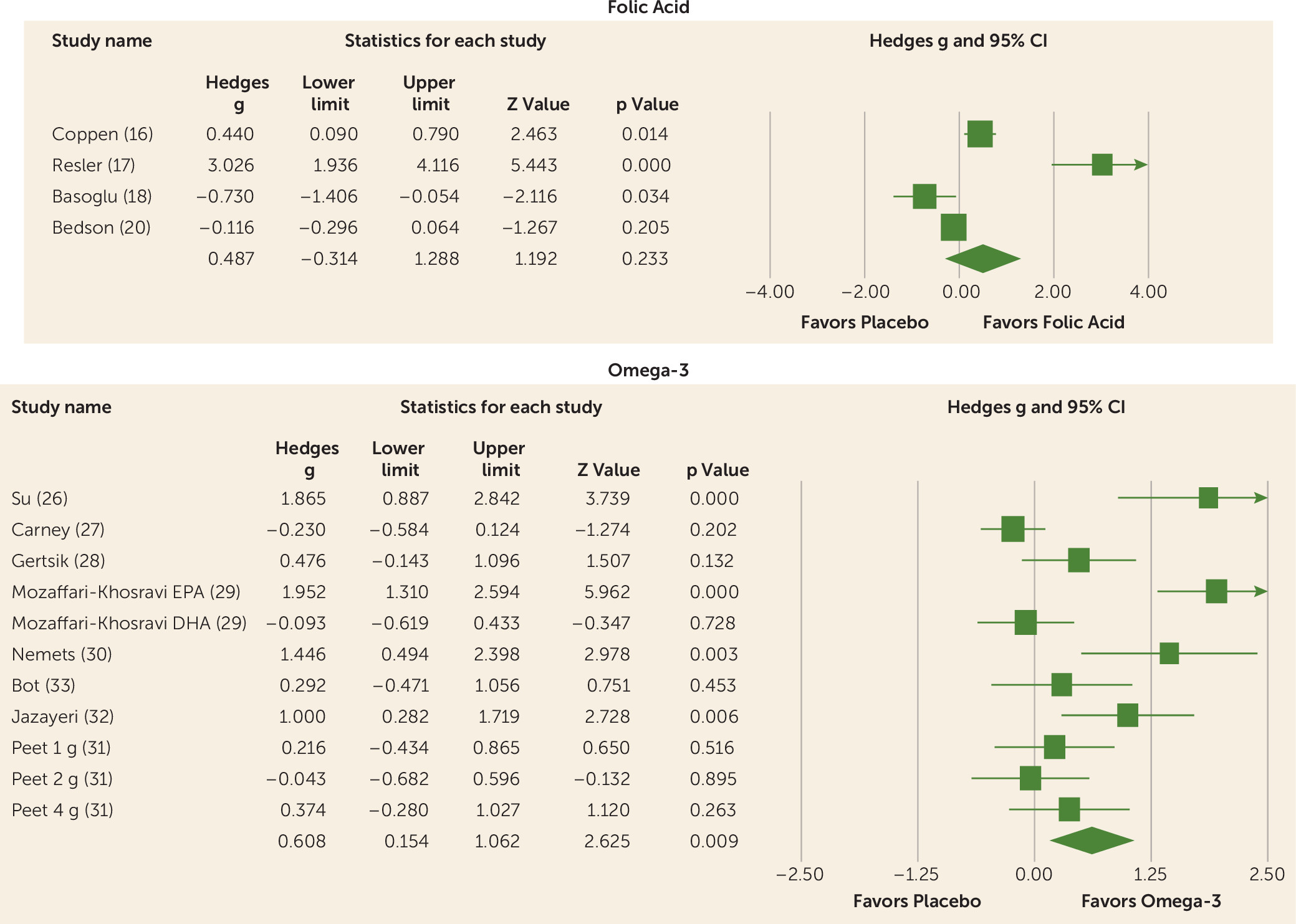
Omega-3
Tryptophan
Other Nutraceuticals
Safety
Discussion
Footnote
Supplementary Material
- View/Download
- 159.95 KB
References
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

