An optimal approach to clarifying the causal interrelationships between pregnancy and drug abuse would require a large, representative, population-based cohort that could be studied longitudinally and in which it would be possible to apply “natural” experimental methods to clarify causal pathways. We report such a study here. Using population-wide Swedish registry data, we evaluated four complementary methods to elucidate the nature of the relationship between pregnancy and drug abuse risk:
We also explored whether the impact of pregnancy on risk for drug abuse varies as a function of parental education, prior deviant behaviors, school achievement, age, marital status, and drug abuse in the cohabiting father of the child. Finally, using within-individual analysis only, we examined whether the reduction in rates of drug abuse during pregnancy persisted into the immediate postpartum period.
Method
We utilized several different Swedish population-based registers with national coverage, linking them by each person’s unique identification number. To preserve confidentiality, this identification number was replaced by a serial number. We secured ethical approval for the study from the Regional Ethical Review Board of Lund University (no. 2008/409). Drug abuse was identified in the Swedish medical registries by ICD codes (ICD-9: drug-induced mental disorders [292] and drug dependence [304], and nondependent abuse of drugs [305, excluding 305.0]; ICD-10: mental and behavioral disorders due to psychoactive substance use [F10–F19], except those due to alcohol [F10] or tobacco [F17]). Drug abuse was identified in the Suspicion Register by codes 3070, 5010, 5011, and 5012, which reflect crimes related to drug abuse, and in the Crime Register by references to laws covering narcotics (law 1968:64, paragraph 1, point 6) and drug-related driving offenses (law 1951:649, paragraph 4, subsection 2 and paragraph 4A, subsection 2). Each individual could have several registrations in the criminal (Crime and Suspicion) and medical registers. To avoid double-counting registrations, within each type of register (criminal and medical), we allowed for a 90-day period after each registration in which a new registration was not counted.
To study the association between pregnancy and drug abuse, we selected all females born in Sweden between 1980 and 1990 who had at least one child registered in the Swedish multigenerational register where the mother was likely first aware of being pregnant between the ages of 20 and 35. We assumed an average of 280 days from the end of the last menstrual period to birth, a 28-day menstrual cycle, and the strong suspicion of pregnancy arising 10 days after the missed menstrual period. Therefore, we estimated that women were aware of being pregnant 242 days before birth (280−[28+10]).
We matched each mother to five nonrelated control women with the same year and month of birth. Furthermore, the control woman had to be alive and registered in Sweden at the time of the case woman’s pregnancy and not herself registered as being a mother or having a child within 9 months after the date of birth of the case woman’s child. For all control women, we studied drug abuse during the same period as the case woman (case women, N=149,512; control women, N=747,560). In the next step, we replicated the matching approach, but instead of using nonrelated random individuals as controls, we matched on female cousins and full siblings. In order to achieve a significant number of control individuals, we allowed for up to 3 years’ age difference between the case woman and the relative control woman. We matched 58,640 control cousins to 50,317 case women and 19,812 control siblings to 19,115 case women. By matching on cousins and siblings, we account for a number of unmeasured genetic and environmental factors shared among cousins and siblings. Finally, we studied drug abuse using a within-individual model comparing a 242-day period before the pregnancy to the pregnancy period.
We used conditional logistic regression, with a separate stratum for each case and her control(s), in which we compared drug abuse in the case individual (i.e., drug abuse during pregnancy) with drug abuse in the controls (i.e., drug abuse during a nonpregnant period). Model 1 was only a crude model, whereas in model 2 we adjusted for midparent educational status (≤9 years, 10–11 years, ≥12 years) and the individual’s school achievement (grade point average in grade 9, usually at age 16; see reference
25).
We then combined the population, full sibling, and cousin data sets and performed two co-relative analyses. The first allowed all coefficients for each sample to be independent. In the second, we modeled the genetic resemblance assuming that it equaled 0 for the population, +0.125 for cousins, and +0.5 for full siblings. We compared this model with the previous model, using the Akaike information criterion (
26). If the second model fit the data well, we obtained improved estimation of the drug abuse–pregnancy association among all types of relatives. In this model, we were also able to extrapolate an odds ratio for monozygotic twins (there was only one monozygotic twin registered for drug abuse while pregnant).
In additional analyses, we investigated whether the association between pregnancy and drug abuse was moderated by the following variables: midparent educational status; school achievement of the individual; lifetime registration of drug abuse in a parent (dichotomized into yes/no); drug abuse registration in the individual prior to the pregnancy period; registration for a psychiatric diagnosis in the inpatient or specialist registries; criminal registration in the individual prior to the pregnancy period (for a definition of criminal registration, see reference
27); age at pregnancy (dichotomized into ≤25 years and >25 years); marital status (dichotomized into married/not married; in the analyses of married people, individuals who got married during the control or hazard period were excluded); drug abuse registration in the child’s father prior to the pregnancy period; and drug abuse registration in the child’s father during the pregnancy period. These moderation analyses were performed only on the within-individual sample using an interaction term between the covariate of interest and drug abuse status in the mother. In the within-individual sample, we also tested whether the decrease in drug abuse rates between control and pregnancy periods was the same for mothers and fathers. This was done by including an interaction term between a dummy variable indicating mother or father, and drug abuse status in the mother and the father.
Finally, using within-individual analyses only, we examined the rates of drug abuse in the immediate postpartum period. Our control was the 242-day period before pregnancy, and we examined three 242-day postpartum risk periods: 0–242 days, 243–484 days, and 485–726 days after childbirth. We eliminated from these analyses mothers whose child died during the risk period. We included mothers who were no longer cohabiting with their child (N=249, N=871, and N=1,187 for the three periods, respectively) because these mothers had substantial elevations in drug abuse rates, suggesting that in some cases the child had been removed because of problematic behavior. Excluding them would bias the postpartum drug abuse rates downward.
In all these models, the within-individual and within-family clustering was taken into consideration. In models that included information on fathers, the father had to cohabit with the mother at the end of the calendar year when the child was born. All statistical analyses were performed in SAS, version 9.3 (
28).
Discussion
We utilized multiple approaches to clarify the magnitude and causal nature of the association between pregnancy and drug abuse in Swedish women. We addressed five specific questions, which we review in turn here with the results.
First, consistent with previous studies (
20,
23,
24,
29), we showed a moderate cross-sectional inverse association between pregnancy and rates of drug abuse. When controlling for parental education and subject school achievement, this association substantially strengthened, suggesting inverse confounding. With these covariates, rates of drug abuse were reduced by 60% during pregnancy.
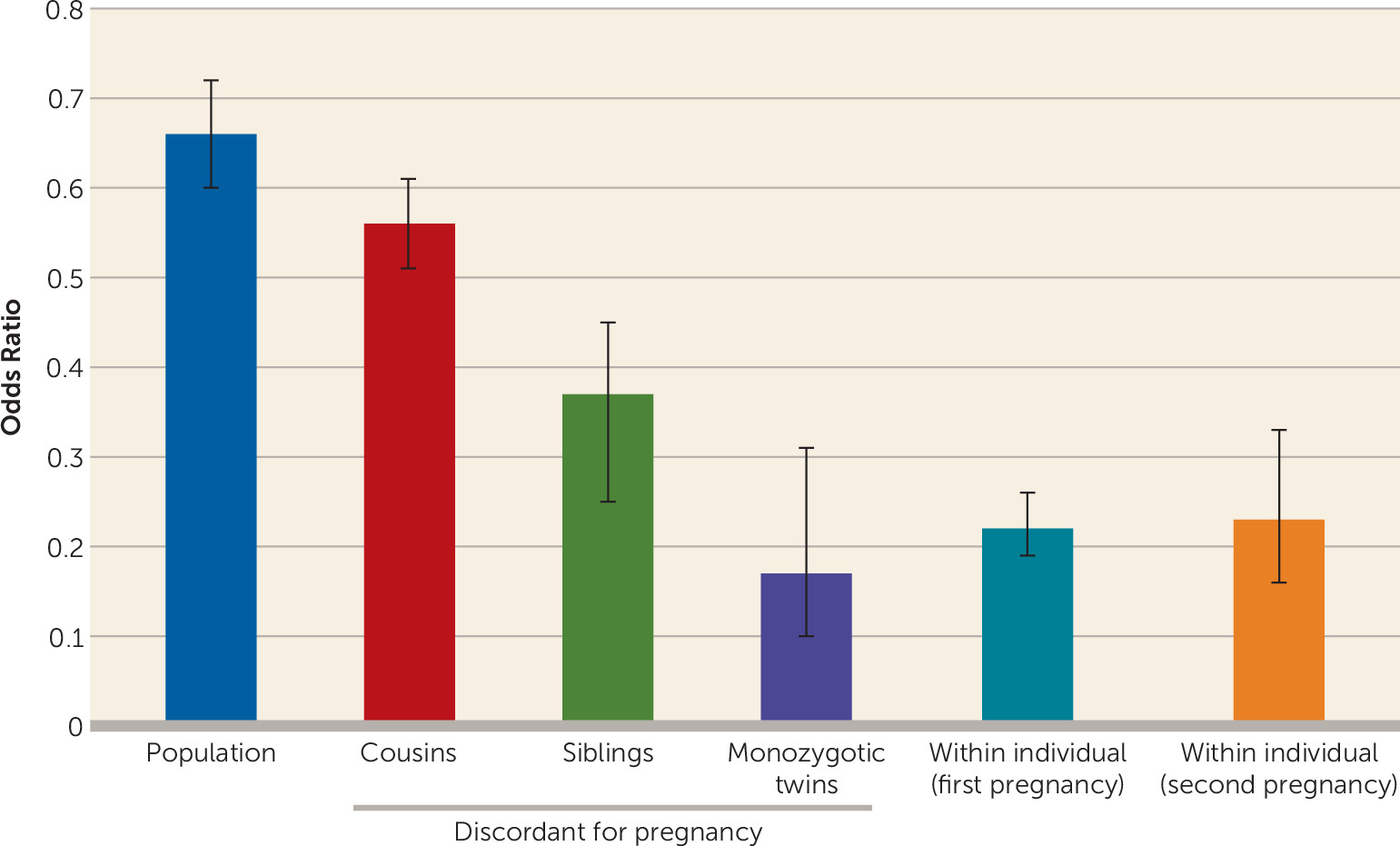
Second, we applied a co-relative design examining risk for drug abuse in pairs of related women discordant for pregnancy. As expected given inverse familial confounding, the pregnancy–drug abuse association became progressively stronger the more closely related the members of the relative pair were. Using a model that fit the data well, we could predict a reduction of 83% in risk for drug abuse in a pregnant woman compared with her nonpregnant monozygotic co-twin.
Third, because pregnancy is episodic and discrete, we could conduct within-individual analyses with subjects acting as their own control. Compared with the immediately preceding equivalent time period, rates of drug abuse declined 78% during pregnancy—nearly identical to the rate estimated in pregnancy-discordant monozygotic twins. We also showed that the protective effect of pregnancy was not affected by changing marital status, was similar in first and second pregnancies, and was stronger in the second half than in the first half of pregnancy. While conclusions from observational data are always tentative, our results from population findings with covariates, our co-relative analyses, and our within-person analyses are consistent in suggesting that the large majority of the inverse association between pregnancy and drug abuse is causal in nature.
These results can be usefully compared with results from several previous studies. In longitudinal analyses of data from the Monitoring the Future study (
22), 4% of pregnant women reported using cannabis in the past 30 days, a rate one-third that of matched nonpregnant women. Multivariate analyses found that most of this effect was independent of other predictors, including marital status. Cross-sectional analyses of recent U.S. national survey data (
29) showed that the rate of recent cannabis use in the second and the third trimester of pregnancy among females 12–44 years of age was 3.6% and 1.7%, respectively, compared with 7.6% in a matched group of nonpregnant women. In a sample of 1,492 consecutive prenatal care patients from four urban U.S. clinics (
23), 55.6% of mothers using illicit drugs reported cessation during pregnancy. In a sample of 1,336 mothers from the Baltimore-Washington Infant Study (
24), 54.6% of mothers who reported illicit drug use quit during pregnancy. Finally, nurse-midwife reports of smoking rates in pregnant women in Sweden (
30) show a decline in both heavy smoking and any smoking, from 12.3% and 21.9%, respectively, prior to pregnancy to 3.3% and 10.1%, respectively, at week 30–32. The validity of these smoking measures is supported by a much higher risk of chronic obstructive pulmonary disease in women still smoking in their third trimester (
30).
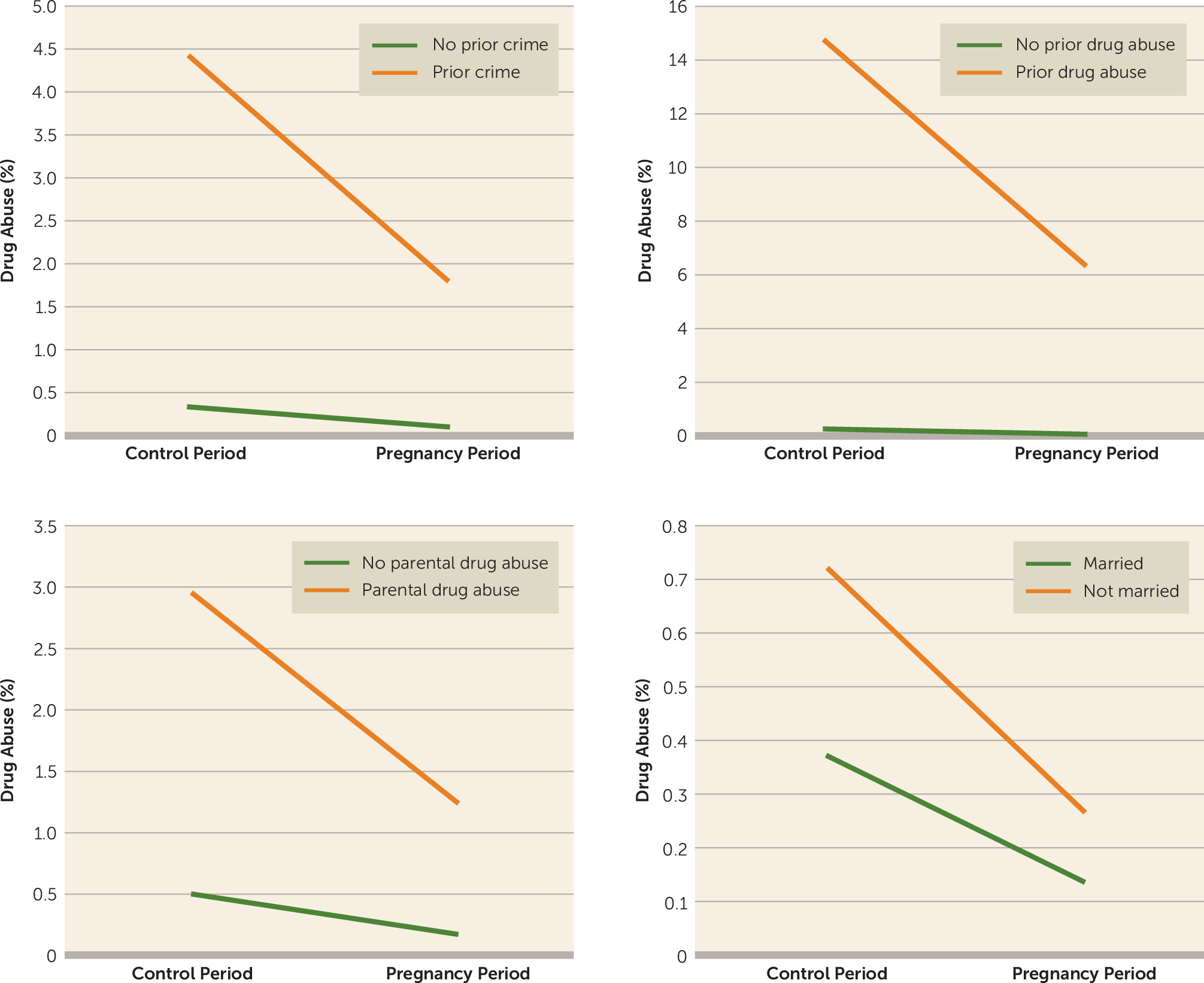
We examined an array of variables that might moderate the impact of pregnancy on drug abuse. For most of them, including prior crime or drug abuse (which strongly predicted risk for drug abuse during pregnancy), the protective effect of pregnancy (as indexed by the odds ratio) was similar in those at low and high baseline risk. However, as shown in
Figure 2, taking a risk difference approach shows that pregnancy reduces the number of drug abuse episodes far more in those at high prior risk than in those at low prior risk. Features of drug abuse in the cohabiting father of the child weakened the protective effect of pregnancy, particularly when the father was abusing drugs during the pregnancy.
Fourth, studying multiple pregnancies and patterns of drug abuse in the cohabiting child’s father provides some insights into the mechanism of the drug abuse–pregnancy association. If it is driven largely by long-term changes in attitudes toward drug use, we would expect an attenuation of the protective effect over multiple pregnancies. If, by contrast, the reduction results from a time-limited desire to reduce fetal exposure, then the effect might be more similar across pregnancies. Our results that show similar protective effects for a first and second pregnancy support the latter hypothesis. The large reduction in rates of drug abuse in the pregnant woman compared with the cohabiting father allows us to isolate the impact of her direct motivation to reduce exposure to her child from more general social pressures on the couple. However, the rate of drug abuse among cohabiting fathers is reduced modestly during their partners’ pregnancy, and fathers who abstain likely encourage the mothers’ abstinence. Indeed, the protective effect of pregnancy on drug abuse risk in mothers weakened when the husband abused drugs during the pregnancy.
Finally, using within-individual analyses, we examined whether the large reduction in risk for drug abuse during pregnancy persisted into the immediate postpartum period. We found that it did, and indeed, during the first year postpartum, rates of drug abuse fell even lower, to 84% below their prepregnancy rates. They then climbed gradually, so that by approximately 2 years after birth, rates of drug abuse were similar to those observed during pregnancy.
Whereas postpartum drug abuse relapse is common in the United States, ranging from 27% for cocaine to 41% for marijuana (
31) and up to 85% for cigarettes (
32), our study showed, after birth, a further decrease in drug abuse below rates observed in pregnancy. Higher rates of breastfeeding among Swedish women (
33–
35) may play a role in this, as breastfeeding appears to be protective against relapse to alcohol (
36) and cigarette smoking (
37). Klee (
38) has reviewed the wide range of factors that might contribute to a protective effect of parenting on drug abuse. More specifically, and consistent with our findings, Bachman et al. (
39), using longitudinal data from the Monitoring the Future study, observed, among both women who remained married and those who remained single, considerable reductions in cannabis and cocaine use associated with the transition to parenthood.
Recent meta-analyses of controlled trials of contingency management in the treatment of drug abuse estimated effect sizes (Cohen’s d) of 0.32 (
5), 0.42 (
4), and 0.58 (
6). Using our within-person analyses, the effect size for pregnancy and early parenthood on drug abuse registration were larger (d=0.83, 95% CI=0.74, 0.92, and d=1.12, 95% CI=1.01, 1.21, respectively), suggesting strong motivation in the mother to protect her developing child and young infant from the direct and indirect effects of drug exposure.
While pregnancy intrinsically motivates many women to discontinue drug abuse, others do not quit. For them, pregnancy provides obstetrics practitioners and counselors with a “teachable moment” to kindle maternal motivation to abstain from drug use (
40). Research finds that contingency management can further encourage prenatal care (
41) and counseling session attendance (
42,
43). Despite some evidence that extrinsic motivation interferes with intrinsic motivation to stop smoking (
44), integrated therapies for drug abuse are gaining support, often pairing interventions that promote intrinsic motivation, such as motivational interviewing, with extrinsic contingency motivation (
44).
These results should be interpreted in the context of five potential methodological limitations. First, our results are limited to the Swedish population and may not extrapolate to other countries. Second, drug abuse was ascertained using medical and criminal records, which are not dependent on subject cooperation or accurate recall. Compared with interviews, these methods likely generate both false positive and, particularly, false negative diagnoses. While large interview-based studies of drug abuse prevalence have not been conducted in Sweden, lifetime prevalence of drug abuse or dependence in nearby Norway is only slightly higher than the estimates we obtain using our methods in Sweden (
46). Third, we cannot rule out additional factors, such as counseling from nurse midwives or threats of child removal influencing pregnant women’s decisions to cease drug abuse. However, removal of children from mothers is rare in Sweden; in a recent year, it involved only 0.08% of children 0–3 years of age (
47).
Fourth, because of frequent amenorrhea, drug-abusing women may have a delayed recognition of pregnancy. We therefore reran our within-person analyses assuming that the women did not recognize their pregnancy until 32 days after what would have typically been their last menstrual period. The resulting odds ratio for drug abuse during pregnancy (odds ratio=0.23, 95% CI=0.19, 0.27) was nearly identical to that found in our main analyses.
Fifth, pregnancy could have an impact on our methods of drug abuse ascertainment. To examine this, we repeated the within-person analyses for drug abuse based on our two most common forms of ascertainment. The protective effect of pregnancy on drug abuse was clearly seen but was slightly weaker from medical (odds ratio=0.32, 95% CI=0.26, 0.40) than from criminal registration (odds ratio=0.20, 95% CI=0.16, 0.25). A similar but more striking difference was seen in the immediate postpartum period, when the protective effects were weaker from medical (odds ratio=0.19, 95% CI=0.15, 0.25) than from criminal registration for drug abuse (odds ratio=0.09, 95% CI=0.07, 0.13).