Sir Henry Maudsley wrote in 1879, “Diabetes is a disease that often shows itself in families in which insanity prevails.” Also in the last century leading researchers, such as Kraepelin and Bleuler, discussed whether altered energy metabolism should be part of the disease picture in schizophrenia. During the last few decades, the introduction of second-generation antipsychotic drugs with diabetic side effects (
1,
2) has increased the focus on comorbid diabetes in psychotic disorders. Unfortunately, it has been difficult to disentangle the effect of drug treatment from the disease itself.
In an elegant national cohort study published in this issue of the
Journal, Rajkumar et al. (
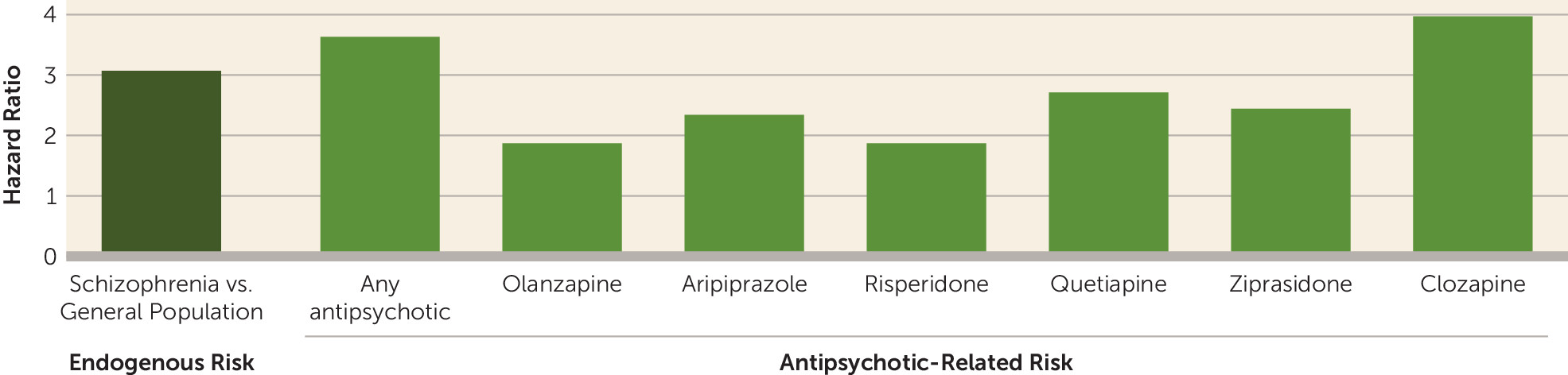
3) show that schizophrenia confers high endogenous risk for diabetes. Before they receive antipsychotics (drug naive), people with schizophrenia have an approximately 3 times higher risk (hazard ratio) of developing diabetes compared with the general population. The risk is further increased 3.6 times (hazard ratio) after the start of treatment with antipsychotic drugs compared with drug-naive patients (see
figure). The data clearly show that diabetes is associated with both schizophrenia itself and with antipsychotic drug treatment. It is important to notice that the increased risk starts from a young age, as early as 15–19 years of age. This highlights the need for earlier detection and more effective treatment of diabetes in people with schizophrenia, which could help improve their physical health.
Why are these findings so important? Several European studies have shown that patients with schizophrenia have about a two to three times higher mortality rate compared with the general population (
4). Patients with schizophrenia have 20% shorter life spans than the rest of the population (
5). A similar alarmingly high reduction of life expectancy is found in the United States (
6). Reducing this gap in life span is a challenge for clinical psychiatry, and the current limited success in reaching this goal is a serious problem for the whole medical field. However, lack of knowledge about underlying mechanisms has limited the ability to find a successful solution to the problem.
It is known that patients with schizophrenia have a strong increase in suicide and fatal accidents, but these unnatural causes of death are very rare. Therefore, more common diseases, such as cardiovascular (
4) and metabolic (
7) disorders, account for the majority of the increased mortality in severe mental illness. The patients’ increased mortality appeared to be caused by an unhealthy lifestyle of smoking, poor diet, substance abuse, and lack of exercise (
4). Although a higher prevalence of diabetes has also been previously reported in schizophrenia (
8,
9), disentangling the secondary and primary (endogenous) relationships has been difficult. Rajkumar et al. (
3) used the large Danish registries to demonstrate that diabetes is associated with schizophrenia independently of antipsychotic drug treatment. Long before antipsychotic drugs became standard therapy, studies showed abnormal glucose tolerance in patients with “dementia praecox” (schizophrenia) (
10). Recent studies of patients in the early phase before initiating drug therapy also show impaired glucose tolerance at the group level (
11). Together, with the data of Rajkumar et al. (
3), these findings suggest that some of the cause of diabetes in severe mental illness is linked to the disease itself (
11).
In addition to lifestyle factors, the causes of the increased incidence of diabetes in schizophrenia can be related to genetic susceptibility. It has been suggested that common genetic vulnerability may predispose to both diabetes and mental suffering. This is supported by findings of genetic variants that increase the risk of diabetes and increase vulnerability to schizophrenia (
12). Overlapping gene variants between schizophrenia and cardiometabolic risk factors have also been identified (
13).
An important aspect of the study by Rajkumar et al. (
3) was the analysis of diabetes risk across individual antipsychotic drugs. No significant difference was found between first- and second-generation antipsychotics. For different drugs the risks (hazard ratios) for diabetes in schizophrenia were found to be 1.88 (olanzapine), 2.35 (aripiprazole), 3.98 (clozapine), 1.88 (risperidone), 2.72 (quetiapine), and 2.45 (ziprasidone) (see
figure). The risk for type 2 diabetes was similar, ranging from 1.94 (olanzapine) and 2.19 (aripiprazole) to 3.25 (clozapine). Because this was an epidemiological study with a naturalistic design, there may be some confounders that could affect the results, particularly related to bias in selection of different drugs and adjusting treatment to reduce side effects. Thus, the findings in individual antipsychotics should be interpreted with caution.
A limitation of the study is the lack of specification of the type of diabetes for most analyses. However, a sensitivity analysis limited to type 2 diabetes was performed. Further, the prescription habits among psychiatrists may vary somewhat between countries and differences in health care systems may also play a role. As Denmark has an excellent public health care system, the problem of comorbid diabetes may be even larger in other countries.
It is of great importance that patients with schizophrenia are included in preventive initiatives to reduce diabetes in the population. This can primarily be done through an awareness of health professionals focusing on the physical health of patients with psychotic disorders (
14). We should be active when it comes to assessment, monitoring, and, not least, measures to improve the situation. There are excellent guidelines describing such efforts (
15).