Anxiety disorders are expressed at peak levels during childhood and adolescence, and this early incidence is highly predictive of adult anxiety disorders (
1). In addition, treatment of children or adolescents with selective serotonin reuptake inhibitors (SSRIs), the first line of pharmacological intervention for anxiety and depression, has been associated with suicidality. This has led to preclinical studies demonstrating that SSRI administration during early postnatal and preadolescent periods can cause long-lasting anxiety behaviors, even into adulthood, in animal models (
2). Mechanistic studies in adult animals have demonstrated a link between anxiety, 5-HT, and brain-derived neurotrophic factor (BDNF), a major neurotrophic factor that undergoes elevated expression during the periadolescent period (
3–
5). For example, SSRI administration increases the expression of BDNF in cortical and limbic brain regions, and BDNF is required for the anxiolytic and antidepressant actions of these agents. BDNF is also a critical neurotrophic factor for the development and function of the 5-HT neurotransmitter system, and mutant rodent models with reduced BDNF signaling display increased anxiety behaviors (
6,
7).
A report by Dincheva et al. in this issue (
8) extends this work by examining the effect of a common human single-nucleotide polymorphism (SNP), BDNF Val
66Met, on the development of the 5-HT system and anxiety behavior and the long-lasting effects of chronic SSRI treatment during a critical adolescent period.
The BDNF Val
66Met polymorphism (rs6265), found in approximately 25% of Caucasians and in larger proportions in Asian populations, blocks the processing and activity-dependent release of BDNF and has been linked to reduced hippocampal volume and executive function and increased susceptibility to anxiety and depressive behaviors (
9–
12). Previous work from the Lee lab at Weill Cornell Medicine has demonstrated that mice with a knock-in of the BDNF Met allele (BDNF
Met/Met) reproduce the elevated anxiety behaviors observed in human carriers (
6). In addition, the ability of fluoxetine to reduce anxiety behavior is blocked in BDNF
Met/Met mice, consistent with reports that the actions of SSRI antidepressants require BDNF (
3,
5).
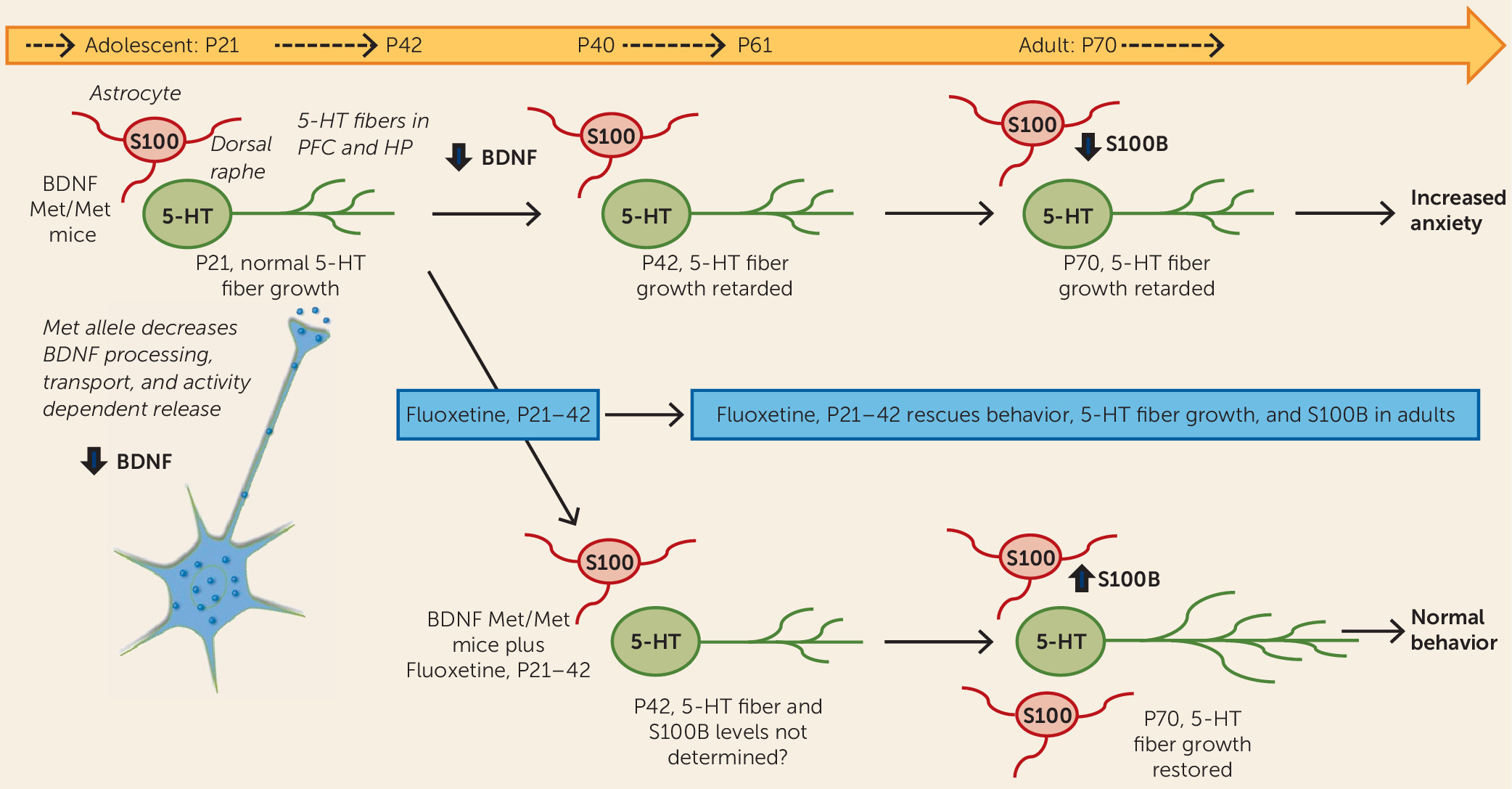
The results of the Dincheva et al. study demonstrate that early adolescent BDNF
Met/Met mice (at postnatal day 30, or P30) display normal behavior, even though anxiety behavior is increased in young adults (at P60), indicating that the impact of the BDNF
Met/Met polymorphism and disrupted BDNF signaling occurs during the adolescent period. Anxiety behaviors are tested in standard models, including the elevated plus maze, in which anxiety is measured as reduced time in the closed versus open arms, and novelty-induced hypophagia as latency to feed in a novel, open environment. These studies were conducted in male mice, but a previous study from this group demonstrates that female BDNF
Met/Met mice show a similar delayed onset of anxiety behavior. Further study by Dincheva et al. shows that fluoxetine administration during an early adolescent phase (P21–P42) rescues the anxiety behavior in the adult BDNF
Met/Met mice (
Figure 1); fluoxetine administration during later adolescence (P40–P61) or in adult mice (P60–P81) had no effect on behavior. Notably, in wild-type BDNF
Val/Val mice, there was no effect of fluoxetine regardless of when in the course of development it was administered. These findings indicate that normal levels of anxiety are influenced by the status of the BDNF Val/Met allele and that fluoxetine administration during a critical developmental period can normalize this behavior in adult BDNF
Met/Met mice. In contrast, deficits in fear conditioning observed in BDNF
Met/Met mice are not normalized by SSRI administration during any of the developmental periods.
Mechanistic studies of the ameliorative effects of fluoxetine showed that the development of 5-HT fiber projections to the prefrontal cortex is decreased between P21 and P42 in BDNF
Met/Met compared with wild-type mice. Surprisingly, administration of fluoxetine during the critical P21–P42 adolescent period, but not during the P60–P81 period, normalized 5-HT fiber density in the prefrontal cortex and ventral hippocampus of adult BDNF
Met/Met mice (
Figure 1); there was no effect of fluoxetine on fiber density at any time tested in BDNF
Val/Val mice. Next, the mechanism by which SSRI treatment leads to increased 5-HT fiber density was examined. Since BDNF
Met/Met mice fail to show an increase in BDNF levels with SSRI administration, the authors examined another factor, S100B, which is produced by astrocytes and is known to have neurotrophic effects on 5-HT neurons. The results demonstrate that levels of S100B are decreased in the dorsal raphe of BDNF
Met/Met compared with BDNF
Val/Val adult mice; notably, fluoxetine administration during the critical P21–P42 period, but not during other periods, also rescued this S100B deficit (
Figure 1).
The results of this study demonstrate several important findings. First, the BDNF Met polymorphism and the consequential reduction in functional release of BDNF results in increased anxiety behavior, decreased 5-HT fiber density, and reduced S100B levels in adult mice and indicate that early adolescence is a critical period for the neurotrophic actions of BDNF. Second, administration of fluoxetine to increase 5-HT tone during this early critical periadolescent period results in a long-lasting rescue of anxiety behavior, as well as deficits in 5-HT fiber density and S100B, in the BDNFMet/Met mice. Notably, fluoxetine had no effect in wild-type mice, consistent with previous reports, although the literature is mixed. Additional studies will be required to replicate these findings and to further characterize the critical period during which fluoxetine produces beneficial long-term effects, as opposed to increased anxiety caused by earlier postnatal SSRI administration. Further studies are also needed to determine the causal relationships between anxiety, 5-HT fiber density, and S100B. For example, is S100B required for fluoxetine rescue of anxiety behavior and 5-HT fiber density in BDNFMet/Met mice? A related question is whether a decrease in S100B during the critical periadolescent period is sufficient to cause anxiety behavior and to decrease 5-HT fiber density in wild-type mice. BDNF influences many neurotransmitter, neuropeptide, and intracellular signaling systems, so there could be other neurochemical deficits that contribute to anxiety behavior and 5-HT fiber density in the BDNFMet/Met mice. It will also be interesting to determine whether fluoxetine increases 5-HT fiber density and S100B immediately after the critical periadolescent treatment period or whether this effect is delayed.
The results of the Dincheva et al. study have important clinical implications, demonstrating that it is essential to optimize the timing of pharmacological interventions to disentangle the potential beneficial versus deleterious effects of SSRI treatment during childhood and adolescent periods. Toward this goal, further studies will be required to define the critical periadolescent period for intervention in primate models. This is particularly relevant for children or adolescent patients who carry the BDNF Met allele, but it could also generalize to anxiety disorders caused by other genetic polymorphisms or environmental conditions (e.g., trauma or stress). Characterization of the discrete critical periods for the anxiogenic versus palliative effects of SSRIs and interactions with BDNF as well as other polymorphisms and environmental factors could lead to pharmacological and genetic interventions to treat anxiety as well as mood disorders in a personalized, patient-specific manner.