Public health consequences of substance use disorders include premature mortality, infectious diseases, and chronic health problems (
1) as well as criminality (
2). In particular, deaths from overdoses of prescription and illicit opioids have increased in many countries, contributing to substantially reduced life expectancy (
3). Additionally, nonfatal overdoses are common. Studies have shown that 30%−80% of persons who use illicit drugs regularly, by injection or by other methods of consumption, have experienced at least one nonfatal overdose (
4). Furthermore, the nonmedical use of prescription opioids has increased (
5) and has been suggested as a risk factor for heroin use (
6).
The most commonly prescribed medications to treat alcohol use disorder are acamprosate and naltrexone (
7), which are associated with reductions in alcohol consumption (
8). For opioid use disorder, the most commonly prescribed medications are naltrexone, buprenorphine, and methadone (
9). The efficacy of these medications in reducing illicit drug use has been shown to be stronger than the efficacy of psychological treatments (
10). Additionally, these medications improve psychosocial functioning (
11). However, their effect on other adverse outcomes, such as suicidal behavior and crime, is uncertain. This is due in part to trials not having sufficient size or follow-up time to examine rare outcomes (
10,
12). Furthermore, many randomized controlled trials have limited generalizability to real-world practice, because they often exclude patients who have comorbid psychiatric disorders (
13).
Here, we used a large population-based cohort of individuals who received prescriptions for medications for an alcohol or opioid use disorder (i.e., acamprosate, naltrexone, methadone, and buprenorphine), and we investigated the association of these medications with suicidal behavior, accidental overdoses, and crime. We used a within-individual design that compared rates of suicidal behavior, accidental overdoses, and crimes for the same individuals during the period when they were taking these medications with the period when they were not. This design accounts for time-invariant factors, such as early environment and genetics, and confounding by indication—namely, that individuals who receive prescriptions for these medications may have background risks that differ from those of persons who do not.
Method
Participants
We examined data for individuals in Sweden who were treated with a medication for an alcohol or opioid use disorder (acamprosate, naltrexone, methadone, or buprenorphine) during the period 2005–2013.
Study Design
A within-individual design (a variant of self-controlled case series), with stratified Cox proportional hazards regression models, was used to examine associations between medications and outcomes, with each individual entered as a separate stratum in the analysis and serving as his or her own control. Therefore, the obtained hazard ratio was adjusted for (i.e., stratified on) all potential time-invariant confounders for each individual (e.g., genes, all factors before the start of the follow-up period, and all factors that did not change during follow-up). In this design, only individuals who changed medication status during the follow-up period contributed directly to the estimate. All other individuals contributed indirectly to the estimate through the effects of other covariates (e.g., age) on the estimate. Consequently, all individuals were included in the analyses and contributed either directly (i.e., individuals who experienced the outcome) or indirectly (i.e., the remaining individuals in the cohort) to the estimate. Because the covariates in this design are time-varying, we did not test for the proportional hazards assumption. This design is increasingly used in pharmacoepidemiological studies in psychiatry (
14), and further details are provided elsewhere (
15).
Measures
Register linkage.
Data on all individuals were collected from Swedish population-based registers with national coverage and linked through each individual’s unique identification number.
Medications.
We extracted information on medications approved in Sweden for treating alcohol use disorder (acamprosate: Anatomical Therapeutic Chemical [ATC] classification system code N07BB03 and oral naltrexone: ATC code N07BB04) and for treating opioid use disorder, mainly heroin use (methadone [ATC code N07BC02], buprenorphine [ATC code N07BC01] and buprenorphine-naloxone combination [ATC code N07BC51]). Information was extracted from the Swedish Prescribed Drug Register, which identifies prescriptions collected (i.e., “dispensed”) from all pharmacies and addiction treatment centers in Sweden since July 2005 (for further details, see the online supplement).
Suicidal behavior.
Suicidal behavior was defined as suicide attempts or deaths by suicide (ICD-10 codes X60–X84). Information on suicide attempts was collected from the Swedish National Patient Register, which includes all admissions to all hospitals as well as outpatient contacts with specialized secondary care. Information on deaths by suicide was collected from the Swedish Cause of Death Register (for further details, see the Methods section in the online supplement).
Accidental overdoses.
Information on emergency department visits or deaths due to accidental overdoses (ICD-10 codes T36–T50, X40–X49, F10.0–F19.0) was collected from the Swedish National Patient Register and Cause of Death Register, respectively. This included accidental poisoning and acute intoxication by alcohol, by illicit drugs, by medications, or by biological substances and excluded intentional self-poisoning (ICD-10 codes X60–X69).
Crime.
Information on arrests was extracted from the Swedish Register of Persons Suspected of Offenses, which includes all individuals arrested for a crime after a complete investigation by police, the customs authority, or the prosecution service (
16). Information on convictions was extracted from the Swedish National Crime Register, including all convictions in Swedish district courts (for further details, see the Methods section in the
online supplement).
Other measures.
Detailed information pertaining to other measures is summarized in the Methods section in the online supplement.
Treatment Periods
Reflecting clinical prescribing practice (
17,
18), treatment periods with acamprosate and naltrexone were defined as a series of collected prescriptions with no more than 15 days between two collected prescriptions. A treatment period started on the date of the first collected (i.e., dispensed) medication and ended on the same date as the last collected prescription in that series (i.e., the last collected prescription that was within 15 days of a previous prescription). If a prescription was collected more than 15 days after the previous prescription, a nontreatment period was considered to have started on the day after the last collected prescription. A new treatment period was considered to have started again on the day of the next collected prescription. Defining the end of a treatment period as the day of the last collected prescription allows for a more conservative estimate of medication exposure, which could result in lower sensitivity (i.e., individuals are classified as nonmedicated when they may be taking a medication) and underestimation of associations.
For methadone and buprenorphine, treatment periods were defined in the same manner but with no more than 8 days between two collected prescriptions. For all medications, single prescriptions were excluded due to uncertainty of medication adherence (for further details, see the Methods section in the online supplement).
We followed the Strengthening the Reporting of Observational studies in Epidemiology guidelines (see the online supplement).
The study was approved by the Regional Ethical Review Board in Stockholm.
Statistical Analyses
Follow-up started on September 1, 2005, or on the date of migration to Sweden, the first day of release from prison, the first day of secure residential homes for juveniles, or the first day of hospitalization. Observations were censored on December 31, 2013, or in the event of death or permanent migration from Sweden. Follow-up was divided into periods of treatment and nontreatment. To account for time at risk, we excluded time spent in prison, secure residential homes for juveniles, hospitals, or interim migration. First, we examined the four primary outcomes: suicidal behavior, accidental overdoses, any arrests, and arrests for violent crimes. Second, we examined arrests for other crime categories: substance-related and nonviolent crimes. Third, we investigated convictions (rather than arrests). We adjusted for age by coding age as a categorical time-varying covariate, with one category for each whole year. Additionally, we used a quadratic function for age to allow for nonlinear effects.
Sensitivity analyses.
To account for the possibility of reverse causality (i.e., that an individual was prescribed medication because of one of the examined outcomes), we performed sensitivity analyses excluding individuals who initiated a treatment period within 3 months after experiencing the studied outcome (e.g., individuals who initiated a naltrexone treatment period within 3 months after being treated for an accidental overdose).
To further examine associations with suicidal behavior, we conducted analyses using the method of suicidal behavior (poisoning or other methods) as outcome. Additionally, we adjusted for concurrent exposure to antidepressants (ATC code N06A) and benzodiazepines (ATC codes N05B and N05C), separately, as time-varying covariates. We then excluded all medication periods with concurrent antidepressant or benzodiazepine treatment (e.g., co-occurring acamprosate and antidepressant treatment). Furthermore, we examined the hazard of suicidal behavior in each cohort by using benzodiazepines as an exposure and suicidal behavior as an outcome in within-individual analyses (for further details, see the Methods section in the online supplement).
To exclude the possibility of polypharmacologic interactions among individuals treated with methadone or buprenorphine, we performed within-individual analyses in which we excluded individuals who were also treated with acamprosate during follow-up assessment (naltrexone was not excluded, because it is contraindicated for concurrent use with opioid medications).
We tested for nonspecific treatment effects (e.g., contact with medical services) by using penicillin (ATC code JC01) and adrenergic inhalants (e.g., albuterol/salbutamol [ATC code R03A]), respectively, as alternative exposures in the methadone and buprenorphine cohorts. The choice of these alternative medications was determined on the basis of theoretical reasons for not being associated with the tested outcomes (for further details, see the Methods section in the online supplement).
Results
In the total population of Sweden, we identified 21,281 individuals who were treated with medications for alcohol or opioid use disorder between 2005 and 2013. Of these, 10,309 individuals were treated with acamprosate, 4,389 with naltrexone, 3,320 with buprenorphine, and 5,449 with methadone. Between 61% and 73% of these subgroups were men. The demographic characteristics of the study population are summarized in
Table 1. The mean follow-up time for participants was 7.6 years (SD=1.9).
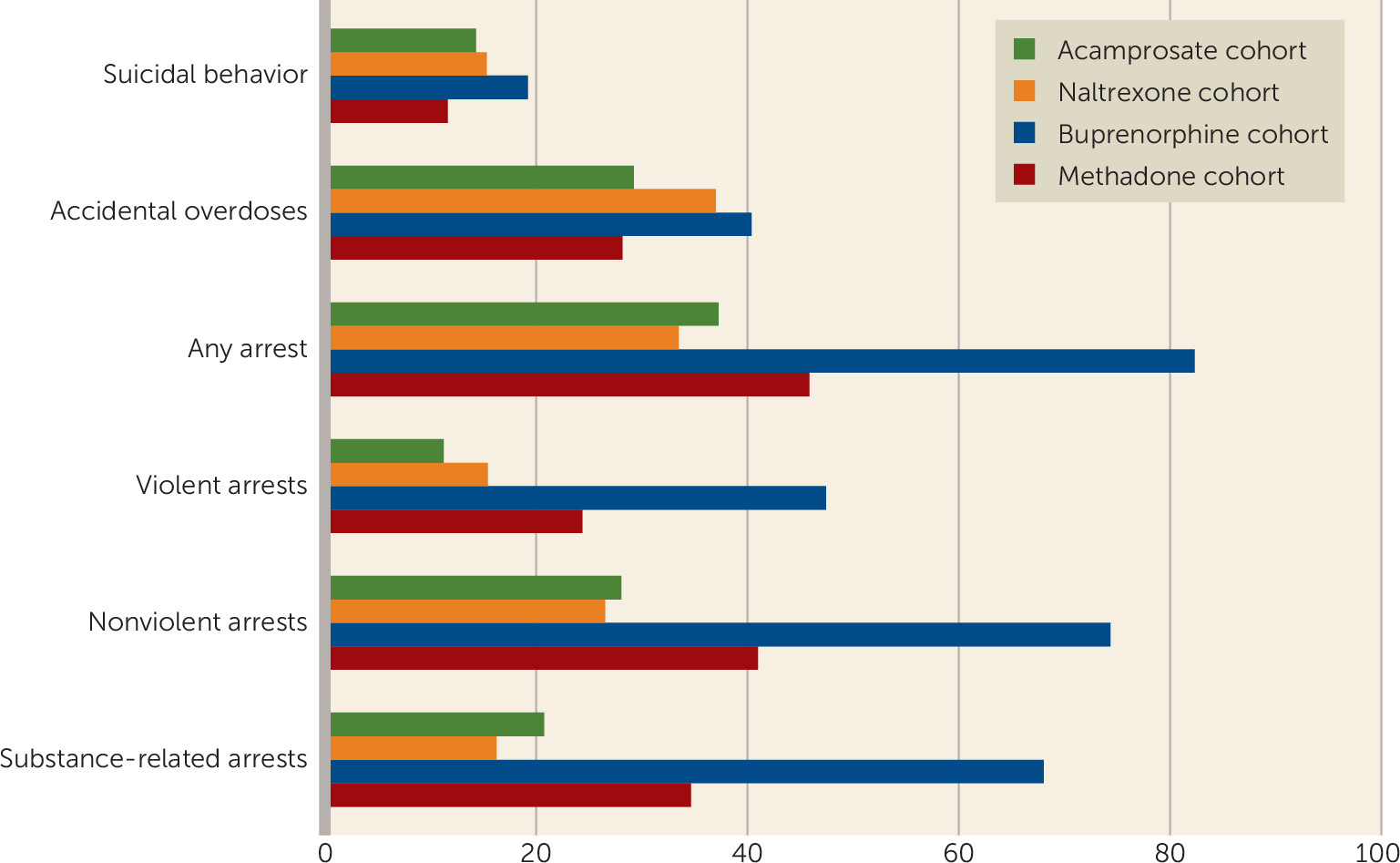
During the follow-up period, 14.3% (N=1,472) of individuals in the acamprosate cohort, 21.0% (N=916) in the naltrexone cohort, 19.3% (N=639) in the buprenorphine cohort, and 11.6% (N=629) in the methadone cohort were treated for or died as a result of suicidal behavior. Furthermore, 29.3% (N=3,025) of individuals in the acamprosate cohort, 37.1% (N=1,626) in the naltrexone cohort, 40.5% (N=1,343) in the buprenorphine cohort, and 28.2% (N=1,536) in the methadone cohort received treatment for or died as a result of an accidental overdose. Additionally, 37.3% (N=3,848) of individuals in the acamprosate cohort, 43.3% (N=1,900) in the naltrexone cohort, 82.5% (N=2,739) in the buprenorphine cohort, and 45.9% (N=2,504) in the methadone cohort were arrested at least once during the follow-up period (
Figure 1).
Associations With Primary Outcomes (Suicidal Behavior, Accidental Overdoses, Any Arrests, and Arrests for Violent Crime)
Acamprosate.
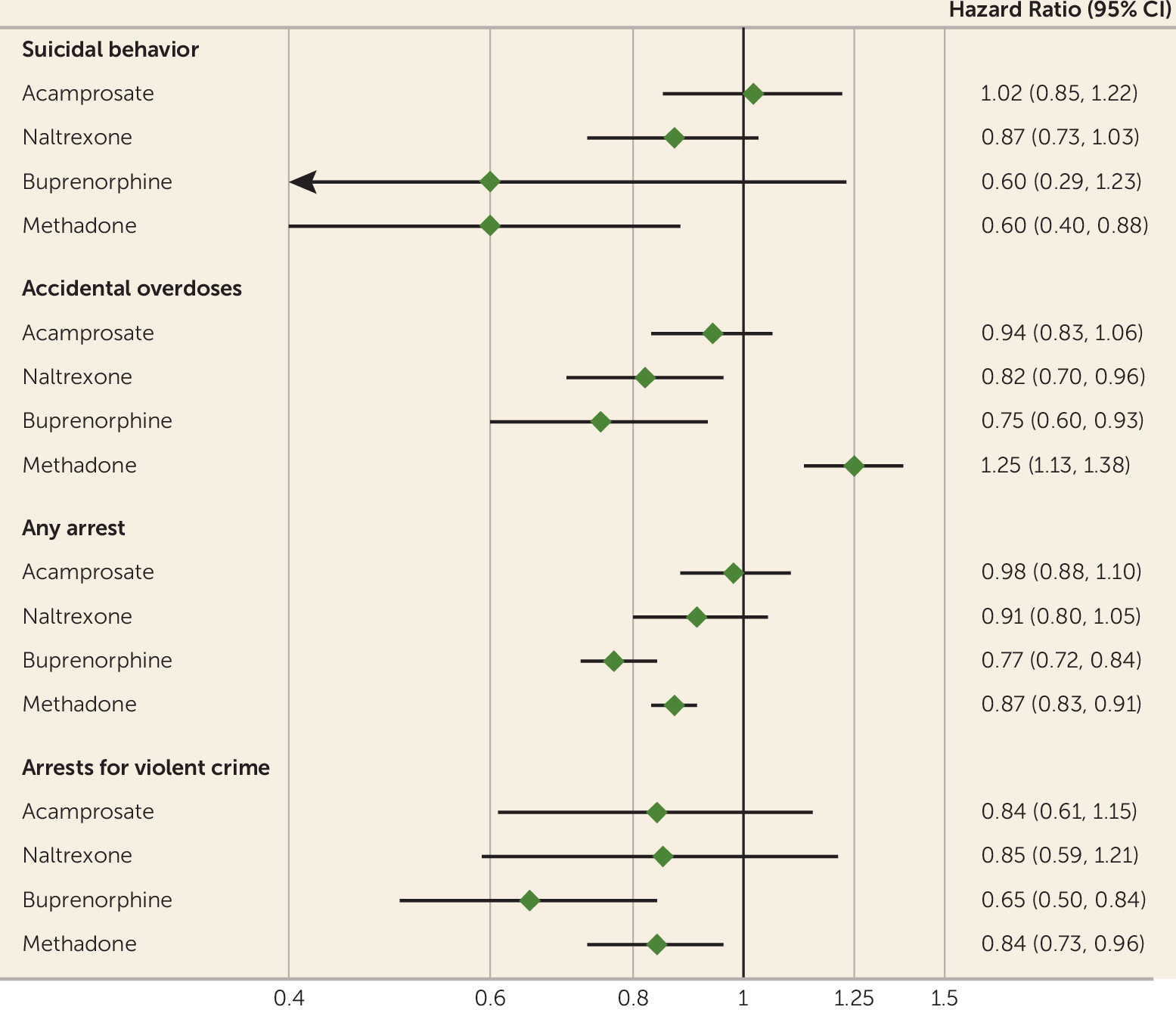
No associations of statistical significance were observed with any of the primary outcomes for acamprosate (
Table 2,
Figure 2).
Naltrexone.
Among individuals who received prescriptions for naltrexone, the hazard ratio for accidental overdoses was reduced during periods when they were taking the medication compared with periods when they were not (hazard ratio=0.82, 95% CI=0.70, 0.96). No other significant associations were found for this medication (
Table 2,
Figure 2).
Buprenorphine.
Significant reductions were found for overall arrest rates with use of buprenorphine (hazard ratio=0.77, 95% CI=0.72, 0.84) as well as for arrests for violent crime (hazard ratio=0.65, 95% CI=0.50, 0.84). A decreased hazard ratio was associated with accidental overdoses (hazard ratio=0.75, 95% CI=0.60, 0.93) (
Table 2,
Figure 2).
Methadone.
Among individuals who received prescriptions for methadone, there was a 40% reduction in suicidal behavior during periods when they were taking the medication compared with when they were not (hazard ratio=0.60, 95% CI=0.40, 0.88). Additionally, significant reductions were associated with overall arrest rates (hazard ratio=0.87, 95% CI=0.83, 0.91) as well as arrest rates for violent crime (hazard ratio=0.84, 95% CI=0.73, 0.96). A significant increased hazard of 25% was associated with accidental overdoses (hazard ratio=1.25, 95% CI=1.13, 1.38) (
Table 2,
Figure 2).
Associations With Secondary Outcomes (Arrests for Nonviolent and Substance-Related Crime and Convictions)
Acamprosate.
Acamprosate was associated with reductions in substance-related convictions (hazard ratio=0.69, 95% CI=0.48, 0.99) but not with other secondary outcomes (
Table 3).
Naltrexone.
Prescriptions for naltrexone were associated with reductions in substance-related convictions (hazard ratio=0.51, 95% CI=0.30, 0.86) (
Table 3).
Buprenorphine.
Buprenorphine was associated with reductions in arrests for nonviolent and substance-related crimes as well as convictions for all crime categories (
Table 3).
Methadone.
Methadone was associated with reduced rates of arrests for nonviolent and substance-related crimes and with lower conviction rates for any crime and for substance-related crime (
Table 3).
Sensitivity analyses.
To account for the possibility of reverse causality, we excluded individuals who started taking their prescribed medication within 3 months after experiencing the studied outcome. When we excluded individuals who were treated with acamprosate within 3 months after experiencing an accidental overdose, we observed a significant decreased hazard ratio (hazard ratio=0.88, 95% CI=0.80, 0.99). In these subanalyses, naltrexone was associated with a significant decrease in suicidal behaviors (hazard ratio=0.80, 95% CI=0.67, 0.95) and overall arrests (hazard ratio=0.85, 95% CI=0.74, 0.97). For buprenorphine, results in the sensitivity analyses were similar to those in the primary analyses. For methadone, the increased risks of accidental overdoses were no longer statistically significant (for further details, see Table S1 in the online supplement).
When examining suicidal behavior further, we first adjusted for concurrent use of antidepressants and then excluded all medication periods with co-occurring antidepressant treatment. Associations remained similar to those observed in the main analyses. When examining the method of suicidal behavior (i.e., poisoning or other) separately, reductions in suicidal behavior remained significant for methadone (for further details, see Table S2 in the online supplement).
We then excluded individuals who were prescribed acamprosate in order to account for the possibility of polypharmacologic interactions among individuals taking methadone or buprenorphine. Results remained similar to those of the overall main analyses (for further details, see Table S3 in the online supplement).
We used penicillin and adrenergic inhalants as negative controls to test for nonspecific treatment effects. No significant associations were found for penicillin. For adrenergic inhalants, significant reductions were associated with any crime that resulted in an arrest (hazard ratio=0.71, 95% CI=0.65, 0.78) (for further details, see Table S4 in the online supplement).
We adjusted for concurrent benzodiazepine treatment. Associations remained similar to those observed in the main analyses—that is, methadone treatment was associated with significant reductions, and no other significant associations were found. When all medication periods with co-occurring benzodiazepine treatment were excluded, reduced hazard ratios were no longer significant for methadone treatment, and no other significant associations were observed. We also examined the risk of suicidal behavior in each cohort by using benzodiazepines as exposure and suicidal behavior as outcome. Results showed increased hazard ratios for suicidal behavior among individuals in the acamprosate and naltrexone cohorts who were taking benzodiazepines (acamprosate cohort, hazard ratio=1.54, 95% CI=1.34, 1.76; naltrexone cohort, hazard ratio=1.45, 95% CI=1.28, 1.64). Individuals in the methadone and buprenorphine cohorts showed no significant associations (for further details, see Table S5 in the online supplement).
Discussion
In this population-based study of 21,281 individuals who were prescribed medications for an alcohol or opioid use disorder, we found heterogeneity with regard to the effect of these medications on suicidal behavior, accidental overdoses, and crime. Using a within-individual design that accounted for factors that remain constant for an individual (e.g., genetic risks and early environment), we found significant reductions in the rate of suicidal behavior when individuals were dispensed methadone compared with when they were not. Additionally, we observed reductions in arrests in the methadone and buprenorphine cohorts, and a similar pattern was found for violent and substance-related arrests. No significant reductions in suicidal behavior or arrests were found in the acamprosate and naltrexone cohorts. When investigating accidental overdoses, we found that naltrexone and buprenorphine were associated with reduced rates; however, there was an increased risk associated with methadone treatment.
The reductions in a wide range of adverse outcomes associated with several of the medications could be seen as a proxy for their overall effectiveness in treating substance use disorders. There are three principal implications of these findings. First, they support the potential role of methadone in reducing suicidal behavior among individuals with opioid use disorder. Deaths by suicide and self-harming behavior are a public health priority, and the efficacy of various pharmacological approaches to address these concerns remains uncertain (
19). However, our findings here, which are consistent with a small body of trial evidence regarding the use of naltrexone and buprenorphine (
20,
21), suggest enhancing the timely and appropriate prescription of opioid medications as an important strategy to reduce adverse outcomes related to opioid use disorder.
The second implication is the potential for use of such medications to reduce crime. There is a lack of consistency in the availability and policies regarding how these medications are used in the criminal justice system (
22), even though opioid therapies have demonstrated clear reductions in reincarceration risk (
23) and violent reoffending risk among individuals released from prison (
24).
The third implication lies in the heterogeneity of the findings in relation to accidental overdoses, which may be explained by differences in the mechanisms of the medications. We found increased hazard ratios for methadone, which is a full opioid agonist with no ceiling to its effects on respiratory depression and sedation. Furthermore, methadone blocks the opioid receptors that bind other opioids (
25). However, lower doses may not be sufficient to prevent withdrawal symptoms and cravings, which could result in the use of supplementary illicit drugs, thus increasing the risk of accidental overdoses. It has been proposed that patients receiving lower methadone doses are at heightened risk for relapse (
25) and that mortality is higher in the early phases of methadone treatment (
26). We found reduced associations with accidental overdoses in the buprenorphine and naltrexone cohorts. Buprenorphine is a partial agonist with high affinity to the opioid receptor but weaker opioid effects compared with methadone, and it has a plateau of effect at increased doses and a lower risk of overdose (
27). Naltrexone is an opioid antagonist that blocks opioid activity and thus could decrease the risk of accidental overdoses from opioids. However, case reports have suggested an increased risk of opioid overdoses among patients who “break through” naltrexone’s opioid block with large doses of opioids (
28).
In terms of clinical implications, the possible implications above need to be interpreted in the context of the potential risks of these medications, such as accidental overdoses with methadone. For naltrexone, there is a potential risk of reduced tolerance in opioid users. This could increase the risk of opioid overdose among individuals who discontinue naltrexone treatment and restart opioid use (
29). However, the evidence is inconsistent (
28,
30). Other clinical implications are the need to ensure that individuals are taking the correct dose of methadone and the importance of screening and treating co-occurring substance use disorders. Among individuals at high risk of accidental overdose, buprenorphine could be considered as a first-line treatment (
31). However, methadone has been shown to be superior to buprenorphine in retaining patients in treatment (
32), and replications of our reported finding of increased accidental overdose risk associated with methadone are required before changes to routine practice are recommended.
Strengths and Limitations
The strengths of this observational study include its large sample size, testing across multiple adverse outcomes, and a within-individual design that accounted for confounding by indication and other time-invariant confounders. A traditional Cox model (i.e., a between-individual design) would have been liable to confounding, particularly confounding by indication (
15), even when adjusting for measured confounders.
A number of important limitations need to be considered. The findings are associations, and causal inferences should not be drawn. It was not possible to exclude the potential effect of contact with medical services, such as psychosocial interventions provided at the same time the medications were taken. This may be particularly relevant in relation to lower rates of suicidal behavior in the methadone cohort, since Swedish guidelines recommend monthly nursing appointments for opioid medications. However, the heterogeneity of the findings across different medications and outcomes would argue against health care contact entirely explaining these findings. Swedish guidelines recommend supervision for suicidal behavior for patients treated with acamprosate, yet we found no association between acamprosate and suicidal behavior. Additionally, we found no significant associations for suicidal behavior or accidental overdoses when we used penicillin and adrenergic inhalants as negative controls. However, there were reductions associated with any crime with adrenergic inhalants, which could suggest nonspecific treatment effects for this outcome.
Differences in service provision may affect the generalizability of our findings. The rates of illicit opioid use are similar across the United States and Sweden (1.5% of the population in the United States, and 1.8% in Sweden) (
33,
34). However, only 15%−20% of individuals with opioid use disorder are estimated to receive opioid treatment in the United States (
35) compared with more than 70% in Sweden (
36). Results could be affected by a potential bias toward the null due to misclassification of exposures or outcomes. On the other hand, this should reduce the hazard ratios reported and would suggest that our estimates are underestimates. Furthermore, the hazard ratio takes into account the timing of an event rather than the number of events over time.
Even though we applied a more conservative estimate of medication exposure (defining periods of more than 8 or 15 days between collected prescriptions as nontreatment periods), we did not have data on medication adherence. This problem is similar to nonadherence in clinical trials, and thus the within-individual estimate is comparable to an intention-to-treat analysis. However, individuals who take opioid medication receive their daily dose under medical supervision for at least the first 6 months of treatment, ensuring adherence. Furthermore, distinguishing suicidal behavior from accidental overdoses is challenging (
37), and some deaths by suicide may have been classified as accidental overdoses due to uncertain evidence, overlapping risk factors, or stigma (
37). Thus, suicidal behavior could be underestimated, while accidental overdoses may be overestimated.
Suicide risks in the naltrexone cohort may have been diluted because our data did not distinguish whether the indication for treatment was alcohol or opioid use disorder. In Sweden, naltrexone is only approved for treating alcohol use disorder, although a proportion may have been prescribed off-label. Moreover, benzodiazepines are often prescribed to treat alcohol use disorder and are associated with an increased risk of suicidal behavior in epidemiological studies (
38). In our study, adjustment for the concurrent use of benzodiazepines did not change associations between medications and suicidal behavior. Benzodiazepines, however, were independently associated with increased hazard ratios for suicidal behavior in the acamprosate and naltrexone cohorts. These findings require further examination in other settings, because we did not have information on the indication (e.g., alcohol use disorder or anxiety) for the prescription. Finally, the use of official registers may have led to underestimation of true outcome rates and possibly involved selection effects.
Conclusions
In this study of individuals prescribed medications for alcohol or opioid use disorder in Sweden, we found potentially important associations with reduced suicidal behavior and criminality. If validated using other designs, evidence-based pharmacologic treatment has the potential to reduce the substantial burden of morbidity among individuals with substance use disorders.
Acknowledgments
The authors thank Daniel Uppström and Markus Takanen for their assistance with the Swedish guidelines on pharmacological treatment of alcohol and opioid use disorders.