Addictions are a leading cause of disability worldwide. Despite advances in substance use treatment, the effectiveness of most interventions remains highly variable across individuals, and multiple quit attempts are common. While a growing body of research suggests that variability in treatment response is linked to individual differences in neural functioning (
1–
6), the search for brain-based predictors has yet to yield a reliable indicator of future treatment response or abstinence (
7,
8). Identification of brain-based predictors of abstinence not only may expand existing biological knowledge of addiction pathophysiology (which may itself be used to refine existing interventions) but also may ultimately be used to directly inform real-world clinical practice by assignment of patients to therapies based on individual patterns of neural function, or neuromarkers (
7,
9,
10).
In most cases, treatment-oriented neuroimaging studies in addiction and other disorders rely on prospective associations (
1–
4), where the term “predict” is often used inaccurately to refer to correlation or regression (
11,
12). True predictive models, however, require application of the model to novel data (
8,
11,
13–
15). Newly available alternatives, such as machine learning, allow for actual prediction (
9,
11,
12) but have not yet been used to identify pretreatment predictors of abstinence. Nonetheless, research indicates that alterations within well-established neural networks (e.g., frontoparietal, salience, default mode) likely contribute to individual differences in treatment outcomes for cocaine use disorder (
3,
5,
16). For example, functional connectivity strength between the medial prefrontal cortex and the temporal pole, when combined with years of education, has been identified as a predictor of relapse (
5). However, no previous study has used a whole-brain, machine learning approach to identify neuromarkers of future abstinence.
Connectome-based predictive modeling (CPM) (
13,
17) is a machine learning approach for generating brain-behavior models from whole-brain functional connectivity data (“connectomes”). Unlike correlation or regression models, CPM with built-in cross-validation protects against overfitting by testing the strength of the relationship in a novel sample, increasing the likelihood of replication in future studies and thus applicability to other clinical samples (
13). Unlike the machine learning approaches that have previously been used to study addictions, CPM is entirely data driven and requires no a priori selection of networks. It is therefore both a predictive tool and a method of identifying networks that subserve specific behaviors—referred to as “neural fingerprints”—and thus it may also be used to identify novel treatment targets (
13,
17). CPM has previously been used to identify neural fingerprints of IQ and attention using whole-brain functional connectivity data acquired during neurocognitive task performance (
17–
19), but it has not previously been used to predict future behaviors or a clinical outcome.
In this study, we used dimensional CPM to identify neural networks predictive of future abstinence from cocaine by applying CPM to functional MRI (fMRI) reward task data acquired at the start of a 12-week treatment for cocaine use disorder. We further tested the stability of these networks over time and in relation to posttreatment abstinence. Finally, we tested the ability of identified networks to predict treatment response in a heterogeneous replication sample. Based on previous work focusing on selected networks (
5,
16), we hypothesized that increased connectivity within and between medial frontal, frontoparietal, and salience networks would positively predict abstinence.
Results
Associations Between Baseline Variables and Abstinence
Spearman’s correlation analyses indicated no significant associations between baseline clinical variables (years of use, past-month use, methadone dosage) and within-treatment abstinence (p values, >0.05). For comparison with CPM findings, a machine learning analysis (i.e., support vector regression [SVR]) of baseline clinical data was also conducted (details are provided in the online supplement). Because CPM is optimized for neuroimaging data, SVR was selected over CPM for this analysis. SVR incorporating baseline clinical variables did not predict within-treatment abstinence (p>0.05).
Predicting Within-Treatment Abstinence
To control for putative effects of residual motion, CPM analyses were conducted both with and without motion as a covariate, and the two approaches yielded similar results (further details are provided in the
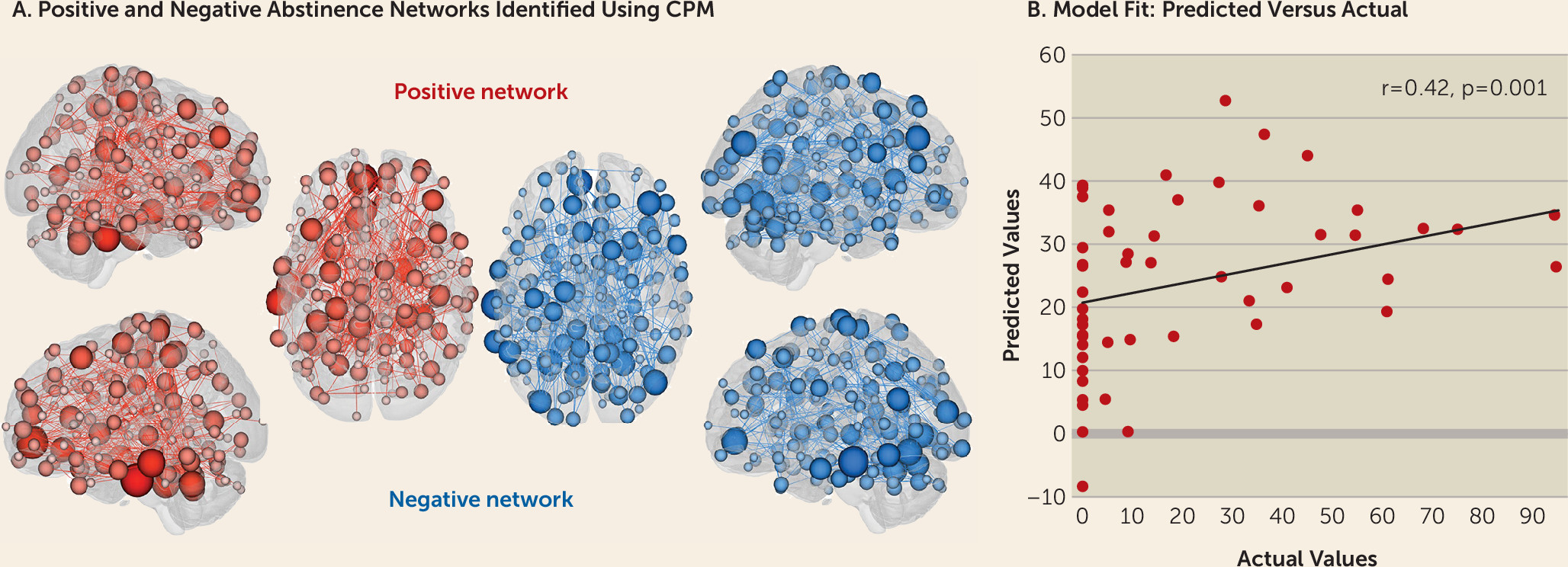
online supplement). For simplicity, findings including motion as a covariate are presented here unless otherwise specified. The overall CPM model successfully predicted abstinence (as indicated by cocaine-negative urine samples) (combined positive and negative networks: r=0.42, df=52, p=0.001) (
Figure 1), as did connectivity within the positive (r=0.43, df=52, p<0.001) and negative (r=0.40, df=52, p=0.003) networks separately.
Follow-up comparisons controlling for methadone dosage, medication group (galantamine or placebo), cocaine use history (years of use, days of past-month use), other drug and alcohol use, smoking status, and timing of fMRI scanning with respect to treatment initiation also successfully predicted abstinence (r values >0.40, with p values <0.003) and are presented in the online supplement. Post hoc correlations indicated significant correspondence between network strengths and other abstinence indices (e.g., percent days self-reported abstinence, maximum days of consecutive abstinence) and are presented in the online supplement.
Network Anatomy
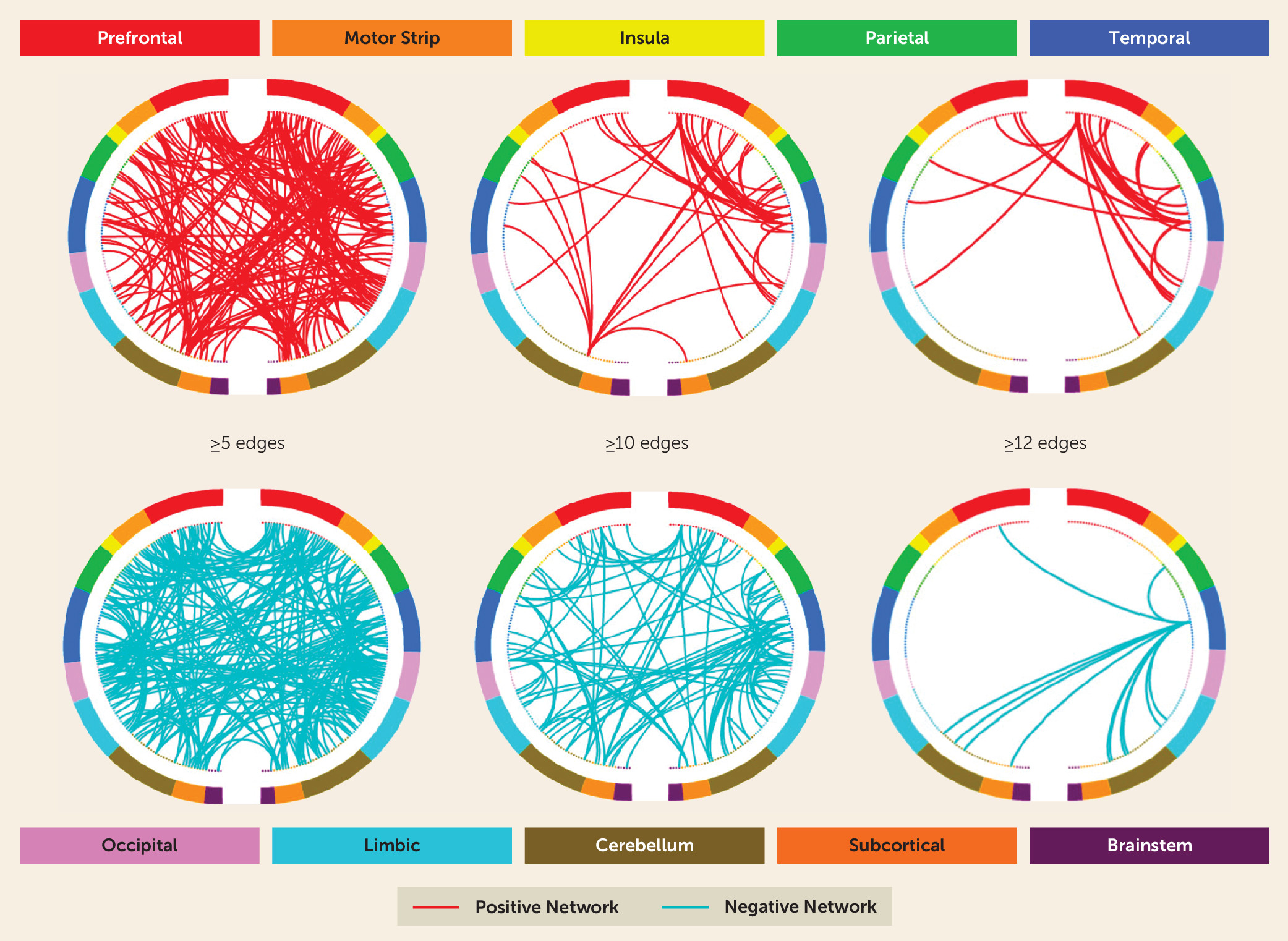
Figure 2 summarizes positive and negative abstinence networks based on connectivity between macroscale brain regions (note that brain regions are presented in approximate anatomical order, such that longer-range connections are represented by longer lines). Consistent with previous CPM work (
17–
19), network anatomies for both networks were complex and included connections between frontal, parietal, occipital, and temporal lobes. Despite this complexity, the spatial extent of both positive and negative networks together included only 529 edges (266 positive, 263 negative), or less than 1.5% of possible connections. Highest-degree nodes (i.e., nodes with the most connections) for the positive network included a prefrontal node with connections to limbic, temporal, parietal, cerebellar, and other prefrontal nodes, and a temporal node with connections to limbic, parietal, motor, and prefrontal nodes. Highest-degree nodes for the negative network also included a temporal node with connections to limbic, parietal, and prefrontal nodes as well as with connections to cerebellar and subcortical nodes. Both abstinence networks included short- and long-range connections. However, the positive network was characterized by relatively more long-range connections (56% long-range; 44% short-range), whereas the negative network included more short-range connections (42% long-range; 58% short-range).
Overlap With Canonical Neural Networks
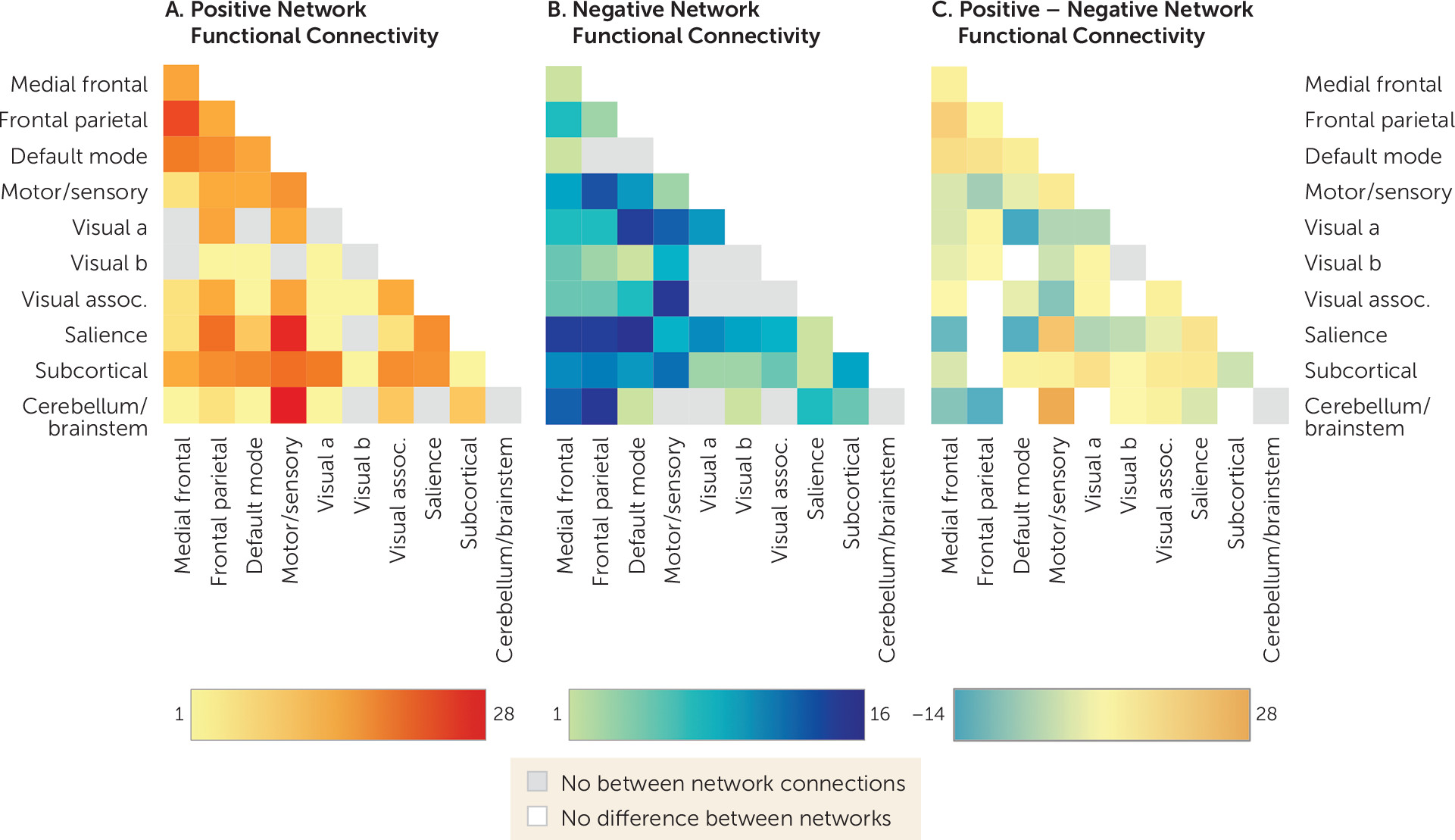
To facilitate characterization of identified abstinence networks,
Figure 3 summarizes connectivity based on the number of connections within and between canonical neural networks (e.g., frontoparietal, motor/sensory) for the positive (
Figure 3A) and negative (
Figure 3B) networks. By definition, positive and negative networks do not contain overlapping connections (as a single edge cannot be both a positive and a negative predictor). However, positive and negative abstinence networks included connections within and between similar large-scale canonical neural networks. Comparison of networks (
Figure 3C) indicated that the positive network included relatively more connections between medial frontal and frontoparietal and default mode networks; between motor/sensory and cerebellar and salience networks; and between subcortical and motor/sensory and salience networks. The negative network included relatively more connections between medial frontal and salience networks; between medial frontal and subcortical networks; between medial frontal and motor/sensory networks; between default mode and salience networks; and between frontoparietal and motor/sensory networks. The positive network was further characterized by more within-network connections across medial frontal, frontoparietal, default mode, motor/sensory, visual association, and salience networks, whereas the negative network included more within-network connections for occipital and subcortical networks.
Relationship to Posttreatment Abstinence
Trial participants were also invited to participate in posttreatment fMRI scanning. After exclusion for excess motion (as described above), 40 participants who underwent posttreatment fMRI scanning were included in the analyses. To determine the extension of our networks to predict abstinence after treatment, individual participant network summary scores were created as the sum of connectivity strengths within positive and negative networks (negative network values were first sign-flipped so that higher values indicated “better” networks). The resultant scores from posttreatment matrices were entered into correlation analyses with abstinence during 6-month follow-up (as defined by self-report using the timeline follow-back method, assessed monthly). Posttreatment connectivity strengths were significantly associated with abstinence during follow-up (r=0.34, df=39, p=0.03). Comparison of pre- and posttreatment networks indicated no significant changes in connectivity strength (t=0.81, df=38, p=0.42).
Out-of-Sample Replication and Binary Prediction
To determine the generalizability of our findings, we tested the ability of the identified networks to predict cocaine-negative urine toxicology outcomes in a heterogeneous sample of cocaine-dependent individuals (N=45). This included previously excluded individuals with excess motion during scanning (N=17) and individuals from an independent, previously published randomized controlled trial (N=28) (
2). As in our original analyses, residual motion was included as a covariate. Further details on exclusion for motion and related analyses are provided in the
online supplement.
Because the practical clinical utility of biomarkers is unlikely to rely on their ability to generate continuous indices of treatment outcome but rather on their ability to identify, a priori, treatment responders from nonresponders in a binary manner, we further tested the ability of the identified networks to predict abstinence in a binary manner (any drug-free urine specimens, yes/no) in our replication sample. Individual participant summary scores were extracted from functional connectivity matrices, as above, and entered into regression analyses with within-treatment abstinence values.
Pretreatment network strength in the independent sample predicted abstinence during treatment for both continuous outcomes (percent cocaine-negative urine samples during treatment; r=0.36, df=44, p=0.016) and binary outcomes (any cocaine-negative urine; 64% accuracy, χ2=5.99, df=2, p=0.014; 82% specificity; 35% sensitivity). For binary prediction, accuracy was increased to 71% after inclusion of baseline cocaine use (days of use in the month preceding treatment) in the model (χ2=6.20, df=2, p=0.02; 89% sensitivity; 41% specificity). As shown in Figure S4 in the online supplement, binarization across different levels of use (≥25% drug-free urine samples, ≥75% drug-free urine samples) decreased sensitivity (57% and 25%, respectively) but increased specificity (71% and 89%, respectively).
Discussion
The translation of brain imaging findings into real-world clinical settings is one of the primary challenges of modern neuropsychiatry (
7–
9,
11–
13). In this study, we demonstrated the ability of a recently developed connectome-based machine learning approach to predict treatment outcomes (abstinence from cocaine during 12-week treatment) using baseline patterns of connectivity. We further demonstrated that posttreatment patterns of connectivity within these networks predicted abstinence during 6-month follow-up. Finally, we demonstrated that the same networks can be used to predict treatment response in an independent, heterogeneous sample. Despite this predictive ability, identified networks could be considered potential treatment targets (
3,
5,
16), and further replication and model refinement are needed before the findings can be directly applied to clinical decision making.
Consistent with the connectome-based approach, abstinence networks were complex and included connections between multiple well-established neural networks (
17,
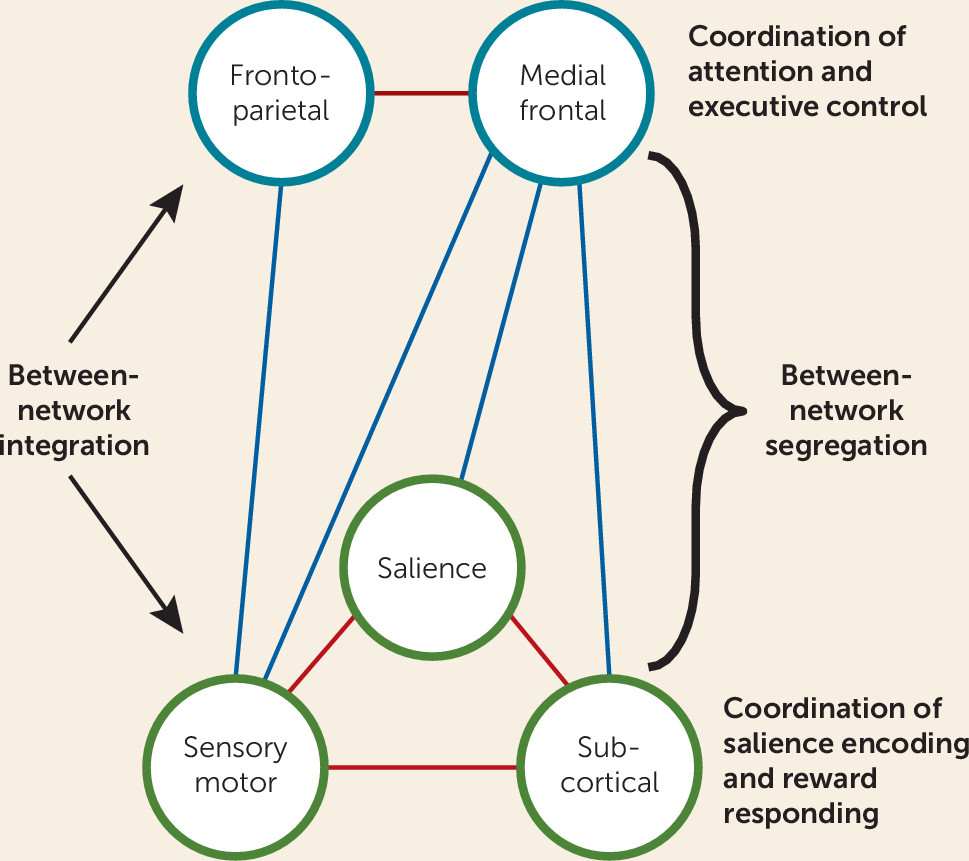
18). The positive network included more frontoparietal–medial frontal–default mode connections as well as more salience–subcortical–motor/sensory connections. In contrast, the negative network (the network for which increased connectivity is negatively associated with abstinence) included more connections between the medial frontal network and salience, subcortical, and motor/sensory networks as well as more salience–default mode connections. Based on these findings,
Figure 4 presents a theoretical network model of abstinence. We propose that abstinence is positively predicted by 1) integration of a cognitive/executive control system involving increased connectivity between frontoparietal and medial frontal networks; 2) integration of a reward responsiveness system involving increased connectivity between salience, motor/sensory, and subcortical networks; and 3) segregation (decreased connectivity) between these two systems. This model builds on previous models of addiction emphasizing separation of frontoparietal and salience networks (
24,
25) but also incorporates medial frontal, motor/sensory, and subcortical networks to provide a theoretical framework for future research.
Within the above context, cognitive/executive control networks are theorized to contribute to abstinence via coordination of top-down processes necessary for treatment engagement (e.g., acquisition of new skills, enhanced control over impulsive behavior), whereas integration of reward networks may support motivational processes relevant to treatment (e.g., willingness to change, attending to alternate rewards) (
3,
26). In addition, based on previous resting-state research in cocaine use disorder (
27), appropriate separation between these two systems is theorized to relate to greater behavioral flexibility (or to decreased compulsivity), as would be required for behavior change during treatment.
Although we did not model specific events of interest but rather used “raw” time courses, our findings are nonetheless intuitive when considered within the context of reward task performance, which requires coordination of both attentional and cognitive control processes as well as of salience encoding and reward response behaviors (
28,
29). These findings are further consistent with recent data prospectively linking medial prefrontal, frontoparietal, and salience networks to cocaine relapse (
5,
16), as well as with data from activation-based studies linking individual differences in brain reward responses to treatment outcomes in addiction (
2,
6,
30). They further suggest that segregation of executive control/attention and salience/reward response systems within the context of performance on the monetary incentive delay task may be optimal for achieving abstinence from cocaine. More generally, these data add to emerging evidence that manipulation of brain states (e.g., via reward task performance) may be helpful in detecting individual differences in brain-behavior relationships (
23). For example, connectome-based predictive models derived from task-based data have consistently outperformed those derived from resting-state data in nonaddicted populations (
31). However, further work across different brain states and in relation to diverse substance use behaviors is needed to test this hypothesis in the specific context of addictions.
Connectivity strength within abstinence networks did not differ between pre- and posttreatment assessments. Previous activation-based studies have demonstrated changes in neural responses after substance use treatments; however, comparatively little is known about network-level changes with treatment. For example, individual differences in connectivity have been found to predict subsequent relapse to cocaine (
5,
16,
32), yet no previous study has compared connectivity before and after treatment for cocaine use disorder. Our findings suggest relative stability of identified networks over 12-week treatment, raising the possibility that abstinence may be more closely linked to pretreatment neural function than to within-treatment neuroplasticity. In this context, it is possible that pretreatment interventions influencing connectivity within the identified networks (e.g., cognitive training, targeted pharmacotherapies) may be helpful in promoting abstinence during treatment (
33–
36). For example, previous CPM work has demonstrated that connectivity strength within networks predictive of attention deficit hyperactivity disorder symptoms is changed after administration of methylphenidate (
19). Thus, it is possible that effective treatments for addiction also influence connectivity within complex networks.
It is further possible that networks contributing to treatment response are distinct from those that are directly implicated in disease pathology or that change with treatment. Brain regions predictive of treatment response in other disorders often have limited overlap with regions consistently found to differentiate patients from control subjects (
37). Clinically, factors that predict treatment response (e.g., motivation to change) may be distinct from those that change with treatment (e.g., acquisition of new skills). Thus, the same may be true for neural networks. Furthermore, it is possible that changes within abstinence networks may take time to emerge and thus may be detectable only after treatment, as would be consistent with data indicating protracted emergence of treatment effects (
38). Additional work is therefore needed to characterize network-level changes over time and in relation to addiction pathology per se. Similarly, future studies should consider how “positive” and “negative” networks change over the course of treatment.
While continuous prediction approaches, which maximize individual differences, are optimal for feature selection in heterogeneous clinical samples (
11), the practical value of predictive modeling within a clinical context will likely involve binary prediction (e.g., identifying treatment responders and nonresponders). We therefore tested the ability of the identified networks to predict categorical outcomes (cocaine-negative urine samples, yes/no) as well as to predict dimensional (percent cocaine-negative urine samples) within-treatment abstinence in an independent, heterogeneous sample of individuals with cocaine use disorder with and without concurrent methadone treatment. Connectivity within the identified networks successfully predicted both categorical and dimensional treatment response in our replication sample.
In our replication sample, our model had high sensitivity but low specificity. In this instance, low sensitivity would translate to underidentification of responders (and thus underassignment of individuals to effective treatment), whereas low specificity would translate to underidentification of nonresponders (overassignment to ineffective treatment). Given that multiple failed treatment attempts are common in addictions—and that only resources are lost in the instance of overassignment to ineffective treatment—maximizing sensitivity in this instance appears paramount.
Strengths and Limitations
This study has several strengths, including use of a recently developed whole-brain predictive modeling approach, multiple-time-point fMRI data (before and after treatment), and out-of-sample replication. Several limitations should be noted, too. Some participants were excluded for missing data or excess motion during scanning, resulting in a relatively modest sample size for our primary analysis (N=53); thus, further work in larger samples is warranted, as noted above. Second, the functional significance of the identified networks in relation to other aspects of substance use pathology remains to be determined. While networks were relatively robust and not significantly changed in follow-up analyses controlling for other factors, we cannot entirely exclude the effects of other clinical variables, such as concurrent use of other substances or even acute intoxication, on connectivity strength. Third, given the relatively limited temporal specificity of urine toxicology analyses, future studies should consider incorporation of salivary testing for acute drug effects. Fourth, further work will be needed to determine generalizability of these findings to fMRI data acquired during performance of different tasks or while the brain is “at rest.” To avoid circularity, we did not test the ability of pretreatment data to predict abstinence during follow-up; however, this will be an important next step for future studies (
5). Consistent with recommendations to encourage model testing in diverse samples (
39), we have made the positive and negative abstinence network masks publicly available at our web site (
https://www.nitrc.org/projects/bioimagesuite/).
Identified networks accounted for just under 20% of the variance in within-treatment abstinence for novel subjects. While arguably somewhat modest, it is important to note that effect size estimates derived from “traditional” statistical approaches—that is, statistics applied to test explanatory hypotheses—are typically larger than those derived from machine learning approaches—that is, statistics applied to predict unknown information (
17,
40). Predictive models are less likely to overfit a specific data set, leading to both increased likelihood of out-of-sample replication as well as typically decreased (more realistic) effect size estimates (
12,
41,
42). Similarly, small effect sizes are found in mega-analyses with ∼10,000 subjects (e.g., findings from the ENIGMA project).