Major depressive disorder is a chronic illness that affects one in six adults in the United States during their lifetime (
1). It is most commonly treated with primarily monoaminergic antidepressant medications, with over 29 million prescriptions written annually in the United States alone (
2). Despite this widespread use of antidepressants, the average effect size for an antidepressant compared with placebo is small (Cohen’s d, ∼0.3) (
3–
6). This small average effect may arise, however, from large effects of medication compared to placebo for some patients, but small or nonexistent differential effects for others. Determining why some patients benefit from antidepressants while others do not is therefore critical for advancing our biological understanding of depression treatment, developing generalizable brain-based principles for treatment development in psychiatry, and, more practically, for our ability to stratify patients on the basis of the likelihood of a positive outcome. At present, however, little is known about biological mechanisms that moderate outcomes between antidepressants and placebo.
Patients with major depression exhibit marked disturbances in mood, affect, neurovegetative function, cognition, and psychomotor activity (
7)—a broad range of capacities with diverse neurobiological underpinnings. The neurocircuitry dynamics underlying these impairments are suggested by abnormalities in major depression in functional networks involved in the processing of both positive and negative emotional information, attribution of salience, and both cognitive and emotional control (
8–
10). Indeed, dysfunction of large-scale networks mediating these functions is seen not only during task engagement but also at rest (
9,
11,
12). Meta-analyses have noted consistent patterns of both resting-state hypoconnectivity (e.g., within the executive control network [ECN]) as well as hyperconnectivity (e.g., within the default mode network [DMN]) (
8,
13,
14). Consistent with dysfunction of a regulatory relationship between these networks (
15), aberrant ECN-DMN hyperconnectivity has also been found in meta-analyses (
8). Thus, not only are the connectivity patterns within the major resting-state connectivity networks altered in depression, but so are relationships between networks (
16).
Despite extensive resting-state functional connectivity research in depression and the use of medication treatment for depression, surprisingly little is understood about how patterns of both within- and between-network connectivity underlie the capacity of patients to respond to antidepressant treatment. Previous studies have, for example, implicated low within-DMN connectivity or within–ventral attention network connectivity as predictors of subsequent treatment nonresponse (
11,
17–
19). Dunlop et al. (
20) found that lower summed resting-state functional connectivity of the subcallosal anterior cingulate cortex to the left anterior ventrolateral prefrontal cortex, dorsal midbrain, and left ventromedial prefrontal cortex was associated with remission in patients treated with escitalopram or duloxetine, while the opposite pattern predicted better outcomes with cognitive-behavioral therapy. However, these studies were limited by small sample sizes, did not compare antidepressant medication with placebo, and/or did not systematically examine connectivity across the entire connectome to reveal an unbiased picture of connectivity patterns specifically related to outcome with antidepressant medication. In this study, we sought to determine whether resting-state functional MRI (rsfMRI) connectivity patterns can provide a pretreatment predictive signal that moderates the effect of antidepressant compared with placebo treatment in a large multisite neuroimaging study. We used a data-driven approach that simultaneously considered all connections within and between the major cortical resting-state networks as well as key subcortical regions such as the hippocampus, thalamus, and striatum. Cortical networks were divided into the seven major previously characterized networks, including the ECN, DMN, salience network (SN; in these analyses, SN is a combination of the original SN as defined by Seeley et al. [
21] and the cingulo-opercular network as defined by Dosenbach et al. [
22]), dorsal attention network (DAN), limbic network (LN), somatomotor network (SMN), and visual network (VN) (
23,
24). The striatum was likewise divided into subregions based on membership in these cortical networks (
25).
Participants in this study were recruited as part of the Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care (EMBARC) study, which is a longitudinal multisite randomized double-blind placebo-controlled trial. A total of 309 patients were recruited into EMBARC, were assessed medication-free prior to treatment, and then were randomly assigned to receive either sertraline or placebo for 8 weeks. As previously described (
26), during stage 1 of EMBARC, participants were started on either sertraline or placebo in matched capsules in a dummy design fashion. A flexible dosing regimen using the measurement-based care approach was used, in which the dosage of medication (number of pills dispensed) was increased according to tolerability and response, with the maximal daily dose of sertraline being 200 mg and that of placebo being 4 capsules.
All participants underwent neuroimaging to identify neurobiological moderators of treatment outcome. Analyses were conducted using an intent-to-treat framework that incorporated all participants, regardless of whether they completed treatment, while controlling for multiple comparisons based on the whole connectome-level false discovery rate. Given the size of EMBARC, its rigorous placebo-controlled design, unbiased statistical analysis, and the fact that rsfMRI acquisition is largely similar across the field, moderators of response to sertraline compared with placebo identified here have the potential to widely inform our understanding of the brain bases of antidepressant treatment, stratify patients by predicted treatment outcome, and more generally promote personalized medicine for the treatment of depression.
Methods
Participants
Institutional review board approval was obtained from each of the four clinical sites (University of Texas Southwestern Medical Center, Massachusetts General Hospital, Columbia University, and University of Michigan), and study procedures were started only after obtaining written informed consent from participants. Details of the design of EMBARC have been published previously (
26). Data for this study are based on participants who were randomly assigned to either the sertraline or placebo arm during stage 1 of the EMBARC trial, which included an 8-week double-blind placebo-controlled trial of sertraline and enrolled 309 participants. (For details on randomization, see reference
26.) Participants were 18–65 years of age, with major depressive disorder diagnosed by the Structured Clinical Interview for DSM-IV Axis I Disorders (
27). (For inclusion and exclusion criteria and their justification, see reference
26.) Briefly, participants had to have a score ≥14 on the Quick Inventory of Depressive Symptomatology–Self-Report at both screening and randomization visits and could not currently be on any antidepressant. Only patients whose first major depressive episode began before age 30, with either a chronic current episode (duration ≥2 years) or recurrent major depression (at least two lifetime episodes) were eligible. Of the 309 participants enrolled, 296 received at least one dose of either sertraline or placebo and underwent at least one baseline assessment (see Figure S1 in the
online supplement for the CONSORT diagram). The EMBARC study also enrolled 40 healthy individuals (
26), two of whom did not have neuroimaging data. Hence, in this report we included neuroimaging data on 38 healthy individuals across the four sites to compare brain signal in major depression to normal response.
Assessment
The 17-item Hamilton Depression Rating Scale (HAM-D) (
28) was the primary outcome assessment tool for this study (
26). In the EMBARC study, the HAM-D was administered at each study visit (at baseline and at weeks 1, 2, 3, 4, 6, and 8 of stage 1). A total score was calculated by summing the 17 items; a higher total score indicates higher depression severity.
MRI Protocol Procedure
MRI scanning was performed on 3-T MR systems. T1 structural and resting-state functional MRI (rsfMRI) data were acquired in the same scanning session. Scans were performed at rest, and participants were instructed to keep their eyes open. The rsfMRI data were acquired via weighted T2* images using single-shot gradient echo planar pulse sequence (TR=2000 ms, TE=28 ms; matrix size, 64×64; voxel size, 3.2×3.2×3.1 mm3; 39 axial slices; and 180 image volumes). (See Table S1 in the online supplement for the acquisition parameter details for each site.)
Functional Image Preprocessing
The rsfMRI data were preprocessed using the Connectivity Toolbox (
29) and SPM 8 (
30). Briefly, the data were preprocessed with slice timing correction, motion correction (realignment and unwarp), spatial normalization to the Montreal Neurological Institute template (matrix=91×109×91, resolution=2×2×2 mm
3), and smoothed with a Gaussian kernel (full width half maximum of 8 mm). Structural MRI scans were segmented into gray matter, white matter, and CSF. Scrubbing was performed using the Artifact Detection Toolbox (
31). Briefly, the Artifact Detection Toolbox was used to detect outliers that met the following criteria: normalized global blood-oxygen-level-dependent (BOLD) signal, z≥3.0; and subject motion threshold ≥0.25. These outliers were used as movement covariates. Physiological and other spurious sources of noise were estimated using the aCompCor method (
32) and used as first-level covariates.
An additional six rigid body parameters (translational and rotational motion) and first-order derivatives characterized each participant’s motion and were also used as first-level covariates. The residual BOLD time series was then bandpass filtered using 0.009 Hz < f < 0.08 Hz to keep only the appropriate frequency fluctuations.
Seed-Based Functional Connectivity Processing
Cortical and subcortical seeds and target regions were selected from various brain parcellations, namely, a 100-brain-region parcellation (
24) and hippocampus (
33), ventral striatum (
25), thalamus, and amygdala (
34) parcellations. The brain parcels were divided into the seven major previously characterized networks: the ECN, DMN, SN, DAN, LN, SMN, and VN (
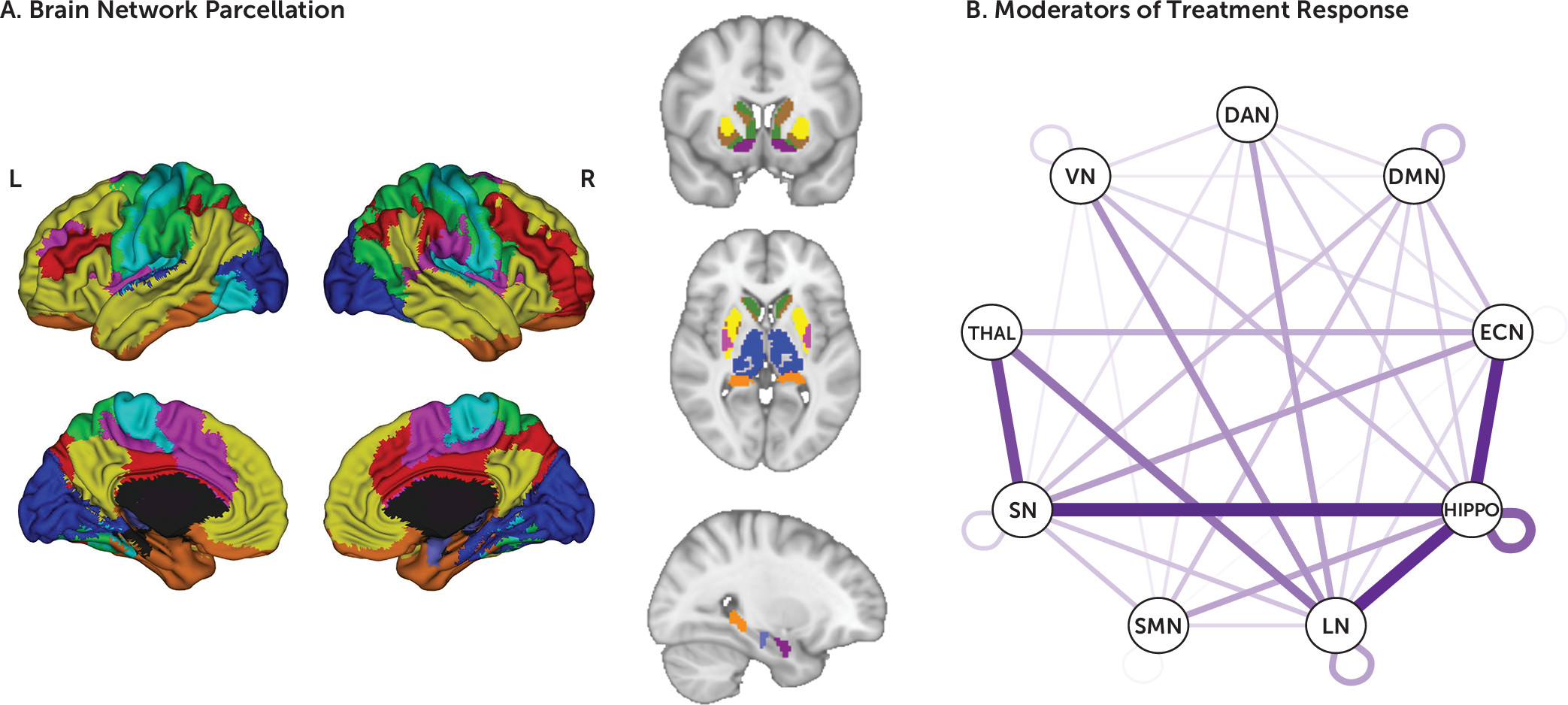
23,
24). The striatum was likewise divided into subregions based on membership in these cortical networks (
25). For visualization, the amygdala was grouped with the limbic network, and the ventral striatum parcels were grouped with each of their respective networks. The hippocampus and thalamus were not grouped with any cortical network.
Region-to-region functional connectivity was also computed using the Connectivity Toolbox. Pearson’s correlation coefficients were computed between the mean residualized, artifact-corrected time courses of the brain regions. These correlations were transformed to a z-score using Fisher’s inverse hyperbolic tangent transformation.
Statistical Analysis
Moderation Analyses.
Intent-to-treat moderation analyses for each region-to-region functional connectivity were conducted using the MacArthur approach (
35) embedded within our longitudinal linear mixed-model analyses. The dependent variable was depression severity at each visit (baseline and weeks 1, 2, 3, 4, 6, and 8), and the independent variables were as follows: functional connectivity, time (i.e., visit), treatment, and site, as well as their interactions (a list of all variables and interactions is provided in Table S4 in the
online supplement). The primary independent variable of interest was a significant treatment-by-time-by-functional connectivity interaction, which suggests that the treatment arms (sertraline and placebo) differed in rate of change of depression severity from baseline to week 8 depending on the functional connectivity of a parcel in reference to the seed parcel. These analyses were performed using the
fmri and
nlme packages in R (
36,
37). To control for type I errors, the F-statistics from the interactions of interest were adjusted using the false discovery rate at p<0.05 in a single test while considering all pairwise connectivity metrics (N=7,260).
To visualize these interactions, we conducted a stratified analysis with participants in the sertraline and placebo arms, respectively. These analyses provided β coefficients that were then utilized to interpret the significant findings from the moderator analyses.
To validate the moderation analyses, we conducted leave-one-out analyses. We repeated this analysis 279 times, each time with one participant excluded. We then computed the correlation between the predicted HAM-D score and the actual exit HAM-D score for each of the moderators that were identified in the overall analysis, yielding a measure of leave-one-out cross-validation for each moderator taken in parallel.
Clinical Meaningfulness
To interpret the moderator effect, we computed effect sizes using the approach outlined by Kraemer (
38), with values ranging from −1 to 1. Higher positive effect size values suggested that higher connectivity was associated with greater improvement with placebo, and higher negative effect size values suggested that higher connectivity was associated with greater improvement with sertraline. We combined multiple moderators using a weighted sum approach as described by Kraemer (
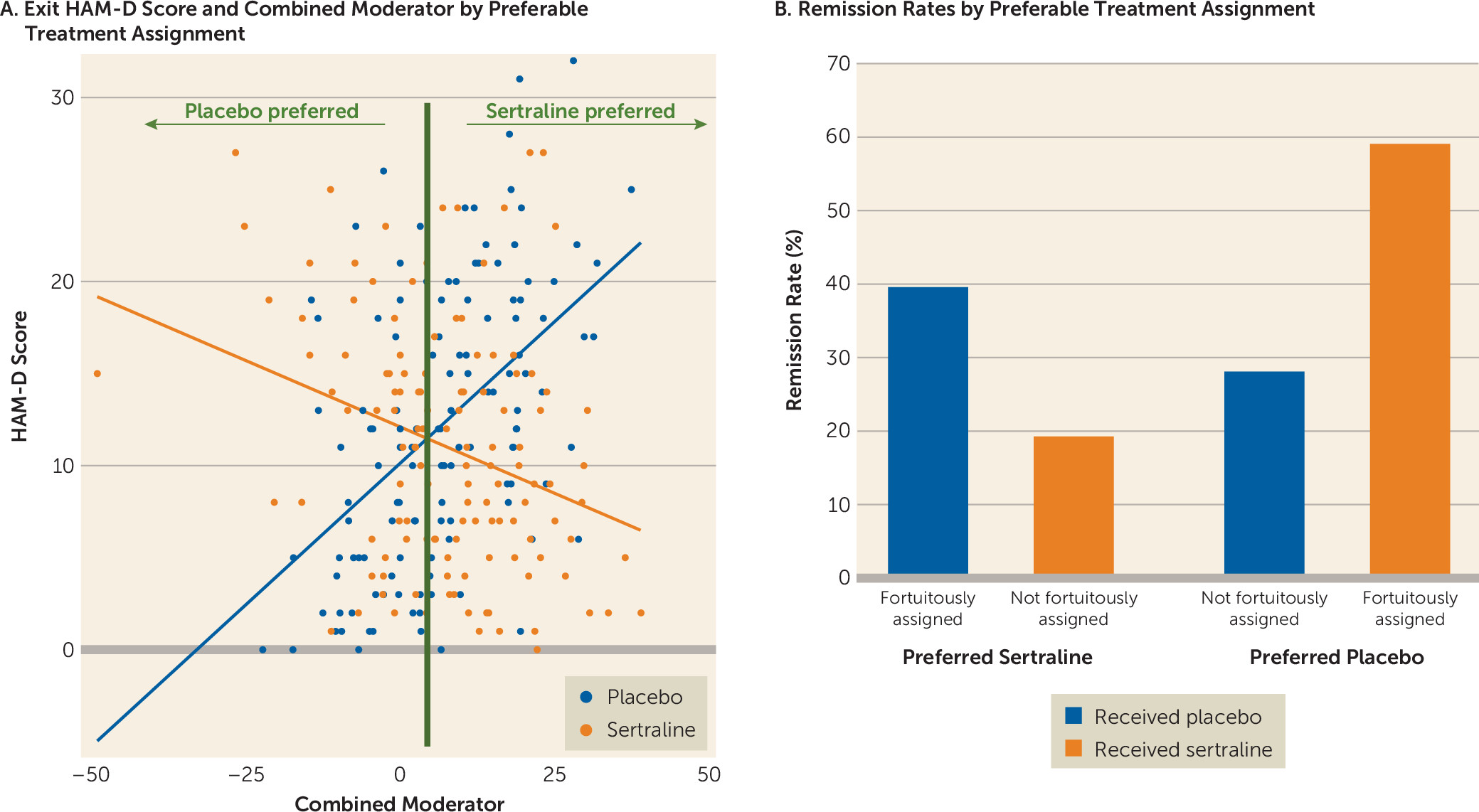
38) to form a composite moderator and computed the effect size. Based on this composite moderator, participants were predicted to respond better to placebo or sertraline. Remission rates according to HAM-D score at the last visit were computed for those who were fortuitously assigned—those who were randomly assigned to their statistically preferred treatment—and those who were not fortuitously assigned—those who were randomly assigned to their non–statistically preferred treatment.
Connectivity Patterns: Depression Group Compared With Healthy Control Group and Association With Illness Severity
To compare the brain signal in the major depression participants relative to the signal in the healthy control participants, we computed the z-scores for those who remitted, for those who did not remit but showed more than a 25% improvement in HAM-D score, and for those did not remit and showed less than a 25% improvement in HAM-D score, and we compared each group’s z-score with the healthy control norm. We also explored the correlations of connectivity pairs identified as moderators with baseline clinical measures of depressive symptom severity and history of childhood trauma. This was to determine whether the moderators were clinically meaningful prior to treatment. These measures include the Altman Self-Rating Mania Scale, the Concise Associated Symptoms Tracking Scale, the Concise Health Risk Tracking–Clinician-Rated Behavioral Module, the Childhood Trauma Questionnaire, the Mood and Anxiety Symptoms Questionnaire, and the Quick Inventory of Depressive Symptomatology (QIDS). Adjustment for multiple comparisons (N=7,260) were performed at p<0.05 using the false discovery rate approach.
Results
Treatment Outcome
Our analytic sample includes EMBARC trial participants who underwent structural MRI and for whom resting-state fMRI data were available that met quality control standards. Among the participants with major depression (N=279), 139 were randomly assigned to receive sertraline and 140 to receive placebo. Of these participants, 32.2% (90/279) achieved remission (defined as a HAM-D exit score ≤7; 49 in the sertraline arm, 41 in the placebo arm), and 62.4% (174/279) participants did not achieve remission (83 in the sertraline arm, 91 in the placebo arm). (See Tables S2 and S3 in the
online supplement for the baseline demographic characteristics of the sertraline and placebo remitters compared with nonremitters.) The remaining 15 participants (seven in the sertraline arm, eight in the placebo arm) exited the study before the first follow-up visit. The mean numbers of visits was 6.0 (SD=1.8) for participants in the sertraline arm and 6.0 (SD=1.6) for those in the placebo arm. See
Table 1 for the demographic and clinical characteristics of the participants. The mean HAM-D score at exit was 11.02 (SD=6.71) for participants in the sertraline arm and 12.34 (SD=12.66) for those in the placebo arm.
Functional Connectivity Moderators
The linear mixed-effects models revealed 138 whole-connectome significant moderation effects after correction for multiple comparisons. Significant moderators were distributed across the connectome, reflecting both within- and between-network connections (
Figure 1). Of these, 112 were between-network moderators (of a total of 6,308 between-network connectivity pairs) and 26 within-network moderators (of a total of 848 within-network connectivity pairs). The largest number of between-network moderators included the hippocampus and the LN; the hippocampus and the SMN; the LN and the thalamus; the hippocampus and the ECN; and the thalamus and the VN. Most of the within-network moderators (18/26) were in the DMN. To visualize the pattern of these moderator connectivity pairs, we organized them into three groups: moderator effect driven predominantly by prediction of symptom change with sertraline and not placebo; moderator effect driven predominantly by prediction of symptom change with placebo and not sertraline; and moderator effects due to significant but opposite-direction predictions of symptom change with sertraline and placebo.
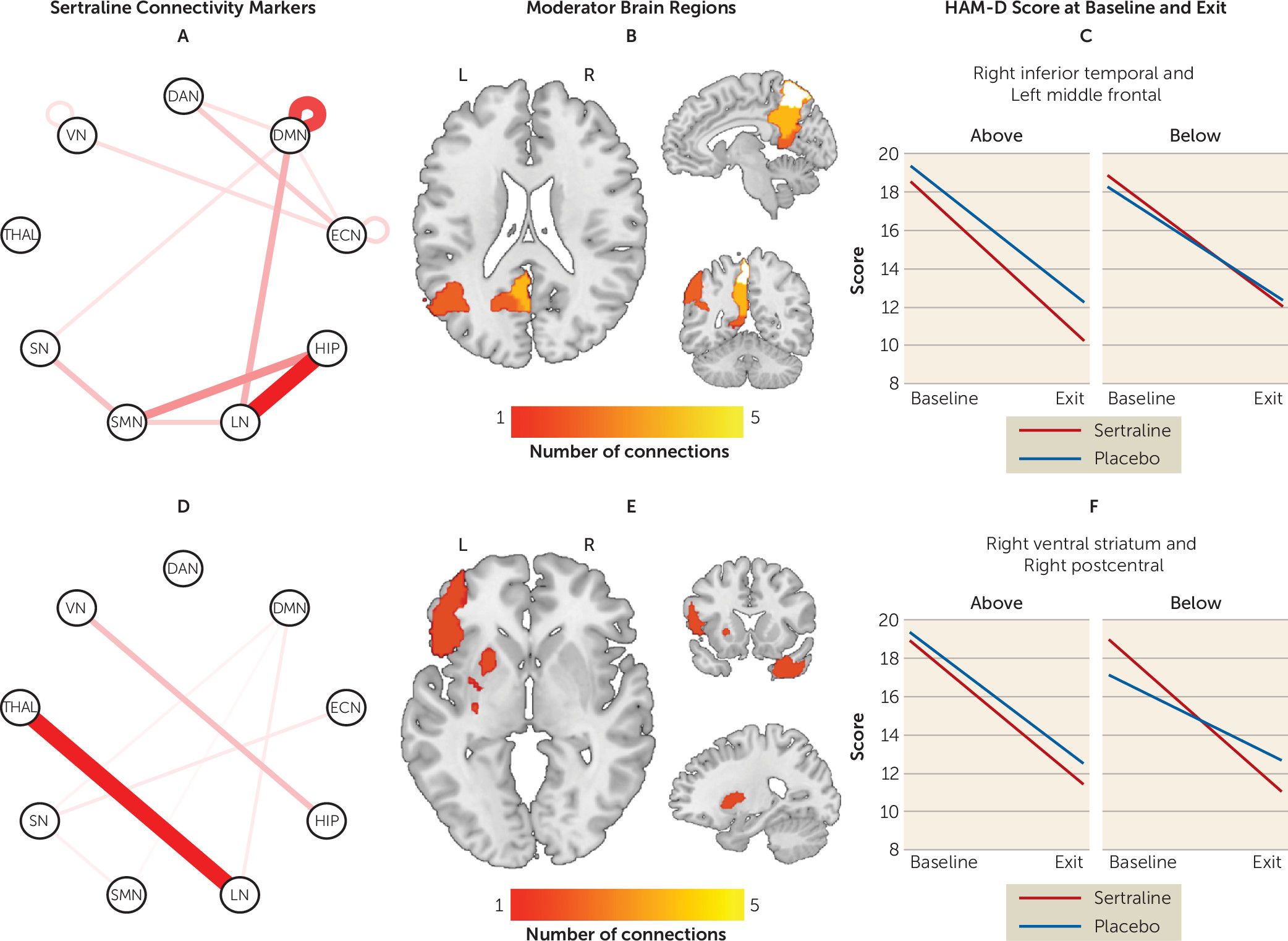
Moderator effects driven by sertraline.
Connectivity patterns that significantly predicted changes in the sertraline arm only (i.e., were noninformative for the placebo arm) are presented in
Figure 2 (see also Table S5 in the
online supplement). Greater connectivity within the DMN predicted better outcomes with sertraline. Between-network connectivity of the hippocampus, LN, SMN, thalamus, and DAN in particular robustly predicted sertraline response. Specifically, higher connectivity of the hippocampus with the LN and SMN predicted a more favorable outcome, as did lower connectivity of the thalamus with the LN.
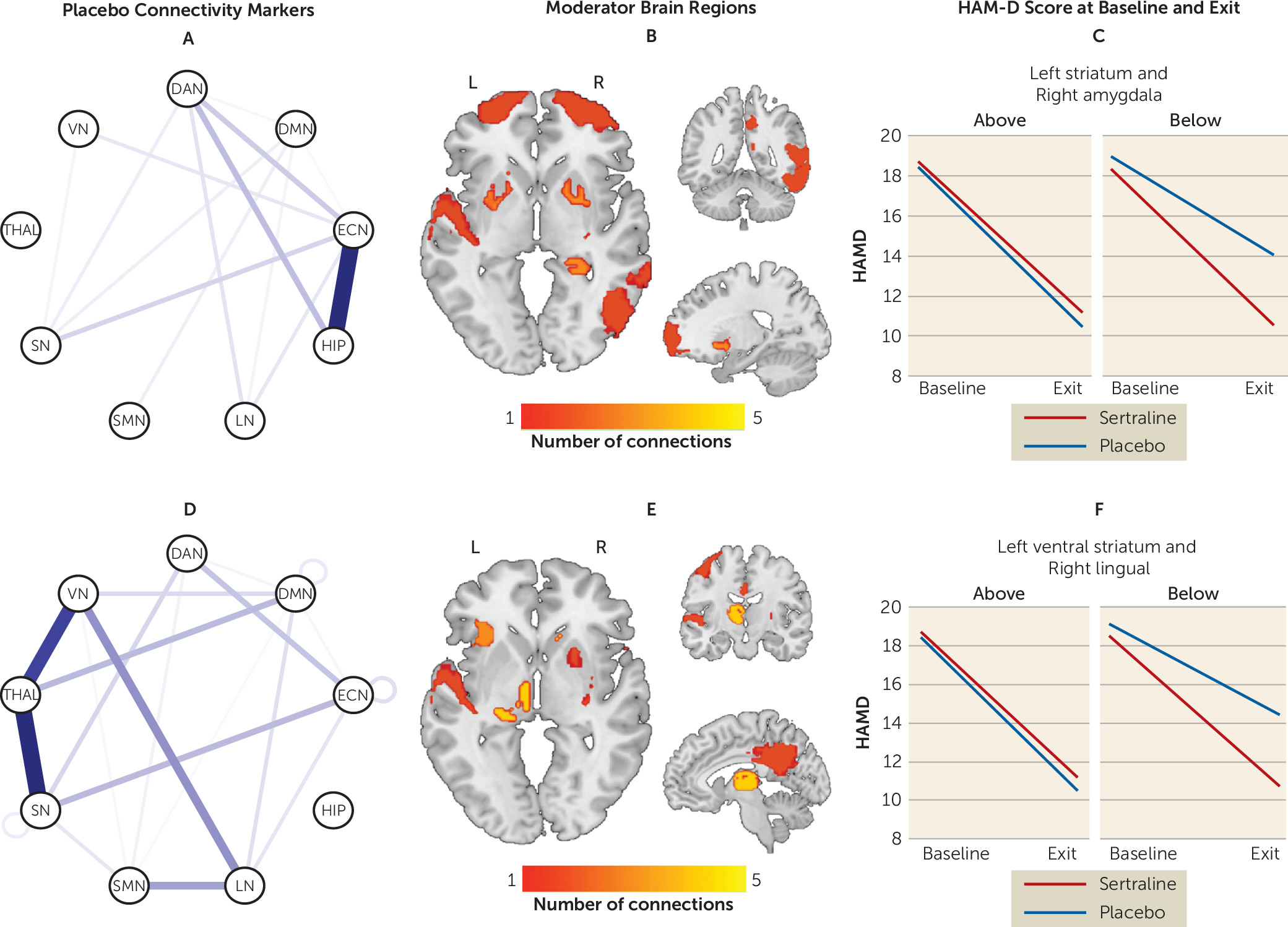
Moderator effects driven by placebo.
In contrast to sertraline, few within-network connectivity patterns predicted better outcomes with placebo (
Figure 3) (see also Table S6 in the
online supplement). In contrast, between-network connectivity of the ECN, hippocampus, thalamus, VN, and SN robustly predicted placebo response. Specifically, higher connectivity of hippocampus with the ECN but lower connectivity of the thalamus with the VN and SN predicted better outcomes with placebo.
Moderator effects driven by opposite prediction in the sertraline and placebo arms.
Connectivity patterns that significantly predicted opposite-direction changes to both sertraline and placebo are presented in Table S7 in the online supplement. Higher connectivity of the DMN and ECN predicted better outcomes with sertraline and worse outcomes with placebo. Higher connectivity of the hippocampus with the VN, DAN, ECN, and thalamus, the LN with the SN and SMN, and the ECN with the SN and SMN predicted better outcomes with placebo and worse outcomes with sertraline.
Leave-one-out validation.
The correlation between predicted and actual HAM-D score for all moderators ranged between 0.22 and 0.36 (all correlations were significant at a false discovery rate-adjusted p<0.05). Correlation coefficients for all moderators are listed in Tables S5–S7 in the online supplement.
Sensitivity and specificity with remission as outcome.
While the goal of this study was to identify neuroimaging biomarkers of differential treatment response using all available treatment visits, we conducted sensitivity and specificity analyses for each connectivity pattern identified as a moderator and have reported these in Table S8 in the online supplement.
Clinical Meaningfulness
The maximum effect size favoring sertraline was seen between the left inferior orbitofrontal cortex and left precuneus, whereas for placebo the maximum effect size was seen between the right precuneus and left striatum. To enhance the clinical meaningfulness of the findings, individual moderators were combined in a linear fashion. The effect size of the combined moderator was −0.40. Based on this combined moderator and, among participants for whom a postbaseline HAM-D score was available (N=263), 192 participants were predicted to respond better to sertraline and 71 were predicted to respond better to placebo (
Figure 4). Of those predicted to respond better to sertraline, remission rates were higher if they were fortuitously assigned and received sertraline (39.33%; 39/99) than those who were not fortuitously assigned and received placebo (19.35%; 18/93). Similarly, of those predicted to respond better to placebo, remission rates were higher if they were fortuitously assigned and received placebo (58.97%; 23/39) than if they were not fortuitously assigned and received sertraline (28.13%; 9/32). The observed prediction rates of the combined moderator reflect the maximal accuracy achievable using the functional connectivity data, given that the assessed outcomes were derived from the same sample used to define the moderators. Replication in separate samples is needed to obtain a truer and more generalizable measure of the accuracy of the combined moderator.
Connectivity Patterns: Depression Group Compared With Healthy Control Group and Association With Illness Severity
There were no significant differences in the connectivity patterns in patients with major depression as compared with healthy control subjects (N=38) who were included in the EMBARC study (
26). For both treatment arms, we grouped major depression participants as those who remitted, those who did not remit but showed more than a 25% improvement in HAM-D score, and those who did not remit but showed less than a 25% improvement in HAM-D score, and we have reported differences in their z-scores relative to the healthy control participants in Tables S9–S11 in the
online supplement. Additionally, there were no statistically significant results (false discovery rate-adjusted p>0.05) for correlations between clinical variables (Altman Self-Rating Mania Scale, Concise Associated Symptoms Tracking Scale, Concise Health Risk Tracking, Childhood Trauma Questionnaire, Mood and Anxiety Symptoms Questionnaire, and QIDS) and connectivity patterns identified as moderators.
Prediction of Treatment Outcomes Irrespective of Treatment
Of the 7,260 connectivity patterns, 259 predicted improvement with both sertraline and placebo, with a false discovery rate-adjusted p<0.05; of these, 70 were within-network connectivity pairs and 189 were between-network pairs (see Table S12 and Figure S2 in the online supplement). The effect sizes of these nonspecific predictors ranged from −0.25 to 0.25. Higher connectivity of the LN with the DMN, SMN, SN, and VN and lower connectivity of the hippocampus with the DMN, SN, and VN predicted better outcomes with both sertraline and placebo.
Discussion
In this large study of depressed outpatients using an unbiased approach, we found a large number of resting-state functional connectivity patterns that predicted differential treatment outcomes between sertraline and placebo. Prediction of sertraline response involved several within- and between-network connectivity patterns. In general, higher connectivity between networks predicted better outcomes with sertraline. The hippocampus emerged as a key hub in connectivity patterns predicting differential response to sertraline and placebo. Higher connectivity of the hippocampus with the thalamus, VN, DAN, and ECN, along with lower connectivity with the SN, predicted better outcomes with placebo. Higher connectivity of the hippocampus with the amygdala (part of the LN) predicted better outcomes with sertraline. Additionally, the DMN emerged as a key network, as higher connectivity within this network consistently predicted better outcomes with sertraline compared with placebo. We also found a number of connectivity patterns that were nonspecific predictors of treatment outcome, that is, they predicted outcomes with both sertraline and placebo. Finally, the individual connectivity patterns can be combined to create a composite moderator that can in turn identify the preferred treatment. We found that patients who received their statistically preferred treatment (based on this post hoc analysis) were significantly more likely to remit as compared with those who did not receive their statistically preferred treatment.
Several connectivity patterns identified in this study are consistent with those reported in previous studies of major depression. We found connectivity within the DMN to be an important network in predicting response to sertraline. Hyperconnectivity within the DMN has been reported in a meta-analysis comparing patients with major depression with healthy control subjects (
8). The association of increased within-network connectivity of the DMN with better response to sertraline suggests that reduction of neural dysfunction in major depression as compared with healthy control subjects may be a potential mechanism of action of antidepressants. Previous studies in healthy adults have found reduced cortical-cortical and cortical-subcortical connectivity with serotonergic antidepressants as compared with placebo (
39,
40). It remains unclear whether this is a serotonergic drug–specific effect, as repetitive transcranial magnetic stimulation has been shown to reduce DMN connectivity in patients with treatment-resistant major depression who exhibited increased within-network connectivity of the DMN than healthy control subjects (
14). While posttreatment neuroimaging scans were not available in EMBARC, indirect evidence for reduction in DMN connectivity with antidepressant treatment may be inferred from a recent study in which first-episode patients with major depression who were taking medications had significantly lower DMN connectivity as compared with those who were not medicated (
41).
The key role of the hippocampus in predicting response to sertraline and to placebo is consistent with its central role in the pathophysiology of major depression (
42–
44). Altered functional connections with the hippocampus may be related to the deficits in emotion-related memory formation seen in major depression (
45). In addition, previous studies have found a negative relationship between functional connectivity of the hippocampus with the prefrontal and parietal cortices in patients with major depression, which was correlated with duration of illness (
46). Previous studies have shown that administration of serotonergic antidepressants reduces the connectivity of the hippocampus with regions in the prefrontal cortex (
47). Whether reduction of connectivity of the hippocampus with the LN and SMN underlies the antidepressant effect of sertraline remains unknown. Connectivity patterns identified in this study did not have a strong relationship with commonly used measures of symptom severity, suggesting that biological markers of illness severity may differ from biological markers of response to antidepressant treatment. The lack of association of increased within-network connectivity in the DMN with illness severity is consistent with a previous report (
48).
An interesting finding of this study was the identification of several regions that demonstrated a placebo-specific response that contrasted with the response to sertraline. As discussed in detail by Fava et al. (
49), there is a need to reconsider the non-drug contributions to the placebo response. Characteristics related to patients, their environment, and the nature of their illness may contribute to non-drug-related improvement that is captured as placebo response in randomized placebo-controlled trials of antidepressant medications.
Given our findings in this study, several next steps suggest themselves: attempting to extend these findings to other treatment modalities; investigating whether there is a relationship between speed of response, remission rates, and level of connectivity (e.g., a person with high connectivity in a brain region might respond faster to a given treatment compared with someone with low connectivity); and using posttreatment imaging to investigate whether these connectivity patterns change with treatment over time.
The limitations of this study should be noted. First, because changes in functional connectivity with treatment were not assessed, the extent to which normalization of impaired connectivity contributes to treatment response cannot be ascertained. Second, the generalizability of our findings to the broad population of depressed patients may be limited by the eligibility restrictions and by the closer clinical tracking that is possible in research studies compared with routine clinical care. Third, although performing rsfMRI in a clinical setting could constitute an advancement in the diagnosis and treatment of major depression, clinical feasibility and implementation may require standardization of automated processing pipelines. Fourth, given that some responders to sertraline may have in fact responded to the placebo effects and not necessarily to the pharmacologic action of the drug, it is possible that our results underestimate the true effect, had the placebo response rate of the population been somewhat lower. Fifth, the scan length (6 minutes) for computing resting-state functional connectivity was relatively brief. While longer scan durations may result in more reliable connectivity data (
50), the duration of the resting-state scan was restricted in EMBARC, to minimize participant burden and to accommodate other neuroimaging tasks. Additional limitations include a lack of detailed information about response to antidepressant medications in previous episodes and too small a sample size in the second stage of EMBARC to explore response to treatments. In sum, the results of this study should be considered preliminary, and replication of our findings in independent samples is necessary for validation.
Conclusions
In this study we identified specific functional network–based moderators of treatment outcome involving brain networks known to be affected by major depression. The connectivity patterns that favored sertraline treatment were 1) greater within-network (DMN) and between-network (DMN and ECN) connectivity of higher-order associative networks; 2) higher connectivity of the hippocampus with the LN and SMN; and 3) lower connectivity of the thalamus with the LN. The study findings demonstrate that measures of intrinsic brain network architecture may play an important role in identifying those individuals who are likely to demonstrate a favorable response for a drug treatment in major depression. Critically, these findings demonstrate that the modest overall effect of antidepressant medication over placebo frequently noted in the clinical literature is not due to small effects of the intervention across all patients, but rather reflects the fact that the depressed population is composed of notable biological heterogeneity—and this heterogeneity determines which patients experience large benefits from medication and those who experience no benefit over placebo.
Acknowledgments
The EMBARC study was supported by NIMH grants U01MH092221 (to Dr. Trivedi) and U01MH092250 (to Drs. McGrath, Parsey, and Weissman). This work was also funded in part by the Hersh Foundation (Dr. Trivedi, principal investigator).