The endogenous neuropeptide nociception/orphanin FQ (N/OFQ) and its target receptor, nociceptive opioid peptide (NOP) receptor, are structurally similar to dynorphin A and its target kappa opioid receptors (
1,
2). However, N/OFQ has no affinity for the mu, kappa, or delta opioid receptors, and endogenous opioids such as endorphins, enkephalins, and dynorphin do not bind to NOP. N/OFQ is an antistress- and resilience-mediating neuropeptide in the brain that counteracts stress and anxiety, which is primarily mediated by corticotropin-releasing factor (CRF), norepinephrine, orexin, vasopressin, and dynorphin (
3). N/OFQ blocks stress-induced analgesia, anorexia, and anxious behaviors in rodents (
4–
6). N/OFQ knockout mice show increased anxiety-like behaviors compared with wild-type mice (
7). N/OFQ also blocks the rewarding properties of cocaine in behavioral paradigms, including the acquisition of conditioned place preference (
8,
9).
In line with this, increases in the rewarding properties of cocaine have been observed in NOP knockout mice (
10). Studies have also shown that N/OFQ can reverse the psychomotor sensitization response that develops following chronic exposure to cocaine (considered to be a rodent model for drug craving) (
9,
11,
12). In addition, N/OFQ reduces cocaine-induced dopamine release in the nucleus accumbens (
12–
14). Two previous studies in rodents evaluated the effects of chronic cocaine on N/OFQ and NOP. In the first study, which used both radioimmunoassay and immuno-autoradiographic techniques, animals that were chronically administered cocaine showed decreased N/OFQ in the nucleus accumbens, substantia nigra/ventral tegmental area, and medial (but not lateral) caudate putamen compared with the control group (
15). In a replication of this observation, the expression of N/OFQ precursor protein (pro-N/OFQ) was found to be decreased in the nucleus accumbens and in the lateral (but not medial) caudate putamen in rodents that were chronically administered cocaine (
16). NOP gene expression (mRNA) examined in the same animals that were chronically exposed to cocaine was significantly increased in the nucleus accumbens (approximately 50%) but decreased in the caudate putamen (approximately 50%–100%), compared with the control group. In summary, the findings from several basic investigations suggest that N/OFQ and NOP alterations modulate drug-induced reward in cocaine use disorders. They also suggest a role for N/OFQ in balancing the functions of the stress-mediating neuropeptides, such as CRF, that promote negative reinforcement and lead to relapse (
3).
Because of the lack of a radiotracer to image NOP, no previous in vivo studies, to our knowledge, have characterized the N/OFQ system in chronic cocaine-abusing humans. [
11C]NOP-1A, first radiolabeled and validated by the National Institute of Mental Health Molecular Imaging Branch, has been successfully used to measure NOP in humans (
17–
19). In the present study, we used [
11C]NOP-1A and positron emission tomography (PET) to measure the in vivo binding to NOP in 24 recently abstinent individuals with cocaine use disorder and 25 healthy control subjects. After the [
11C]NOP-1A PET scan, participants with cocaine use disorder were enrolled in a follow-up protocol in which they were monitored three times per week for 12 weeks using a contingency management approach. The objective of the follow-up protocol was to document relapse and relate it to NOP. We hypothesized that there would be an increase in [
11C]NOP-1A binding in participants with cocaine use disorder compared with healthy control subjects in brain regions that mediate reward and stress behaviors. Such a finding would support the notion that NOP is upregulated in response to decreased N/OFQ in cocaine use disorder.
Results
Twenty-four individuals with cocaine use disorder were matched as closely as possible with 25 healthy control subjects on age, sex, ethnicity, and nicotine use status. The demographic characteristics and clinical variables of the study sample are summarized in
Table 1.
[11C]NOP-1A Scan Parameters
No significant group differences were observed between participants with cocaine use disorder and healthy control subjects in any of the baseline scan parameters (
Table 2). Consistent with previously reported findings, [
11C]NOP-1A plasma free fraction (
fP) measurements were not reliable, because the tracer showed relatively high retention to the filter in the saline buffer solution condition (
24). No significant group differences were observed in the region-of-interest volumes determined from MRI scans.
In Vivo Binding of [11C]NOP-1A (VT)
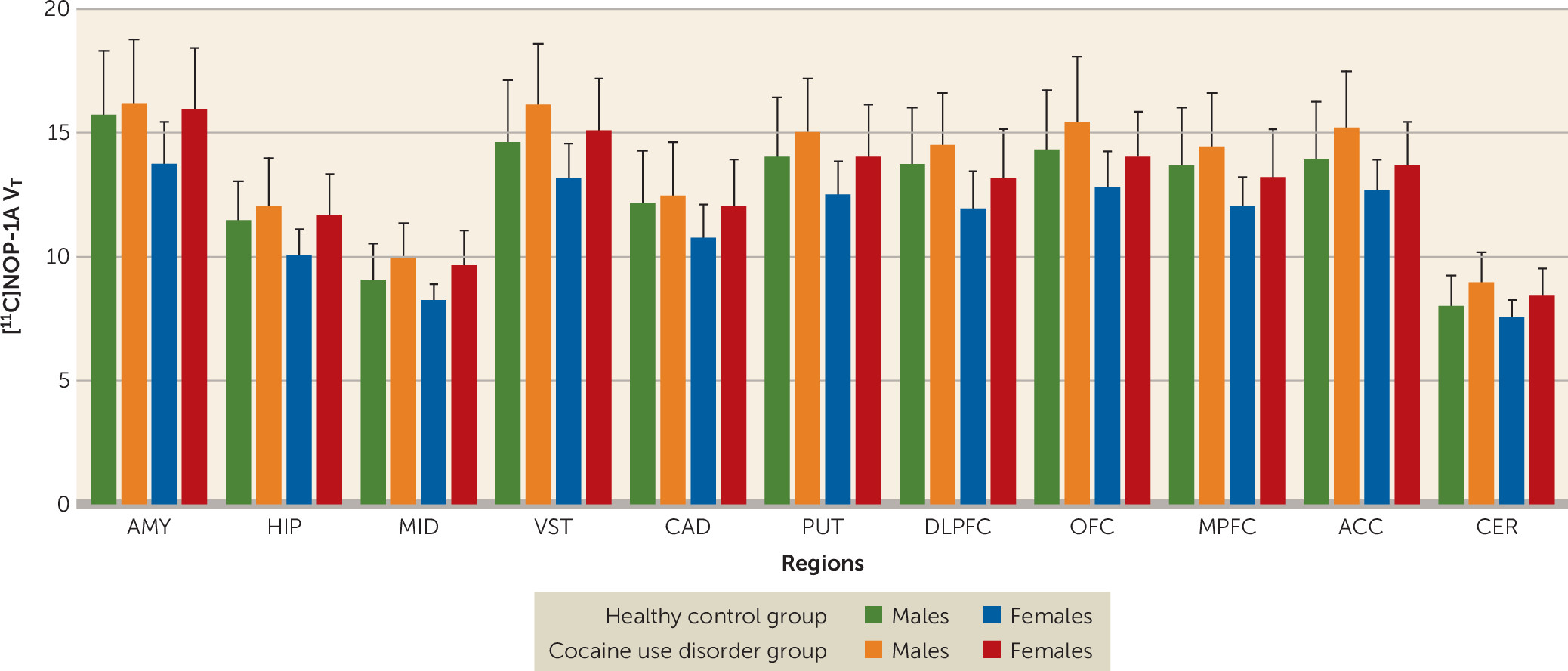
Regional [11C]NOP-1A VT was significantly higher in the cocaine use disorder group compared with the healthy control group (linear mixed-model analysis: effect of diagnosis [F=4.39, df=1, 47, p=0.042], effect of region [F=520.20, df=10, 470, p<0.001], region-by-diagnosis interaction [F=1.45, df=10, 470, p=0.156]).
[
11C]NOP-1A V
T in the regions of interest was on average 7%−12% lower among females compared with males (
Figure 1). The inclusion of sex as a factor in the linear mixed-model analysis did not alter the significance of the result described above (effect of diagnosis: F=4.65, df=1, 45, p=0.037; effect of sex: F=4.45, df=1, 45, p=0.041; sex-by-diagnosis interaction: F=0.25, df=1, 45, p=0.622). However, the effect of diagnosis fell short of significance when tobacco use status was included as an additional factor in the linear mixed-model analysis (effect of diagnosis: F=3.88, df=1, 43, p=0.055; effect of smoking: F=0.03, df=1, 43, p=0.86; smoking-by-diagnosis interaction: F=0.11, df=1, 43, p=0.74). Excluding the four participants in the cocaine use disorder group who tested positive for cannabis during the outpatient monitoring before the PET scan did not change the results (cocaine use disorder group, N=20; healthy control group, N=25; linear mixed-model analysis: effect of diagnosis: F=5.65, df=1, 43, p=0.022; effect of region: F=500.02, df=10, 430, p<0.001; region-by-diagnosis interaction: F=1.97, df=10, 430, p=0.035).
Unpaired t tests conducted at the level of the individual regions of interest were significant in four of the 11 regions of interest (
Table 3). Comparisons in the ventral striatum and cerebellum survived the false discovery rate correction.
Relationship Between VT and Relapse to Cocaine During Follow-Up
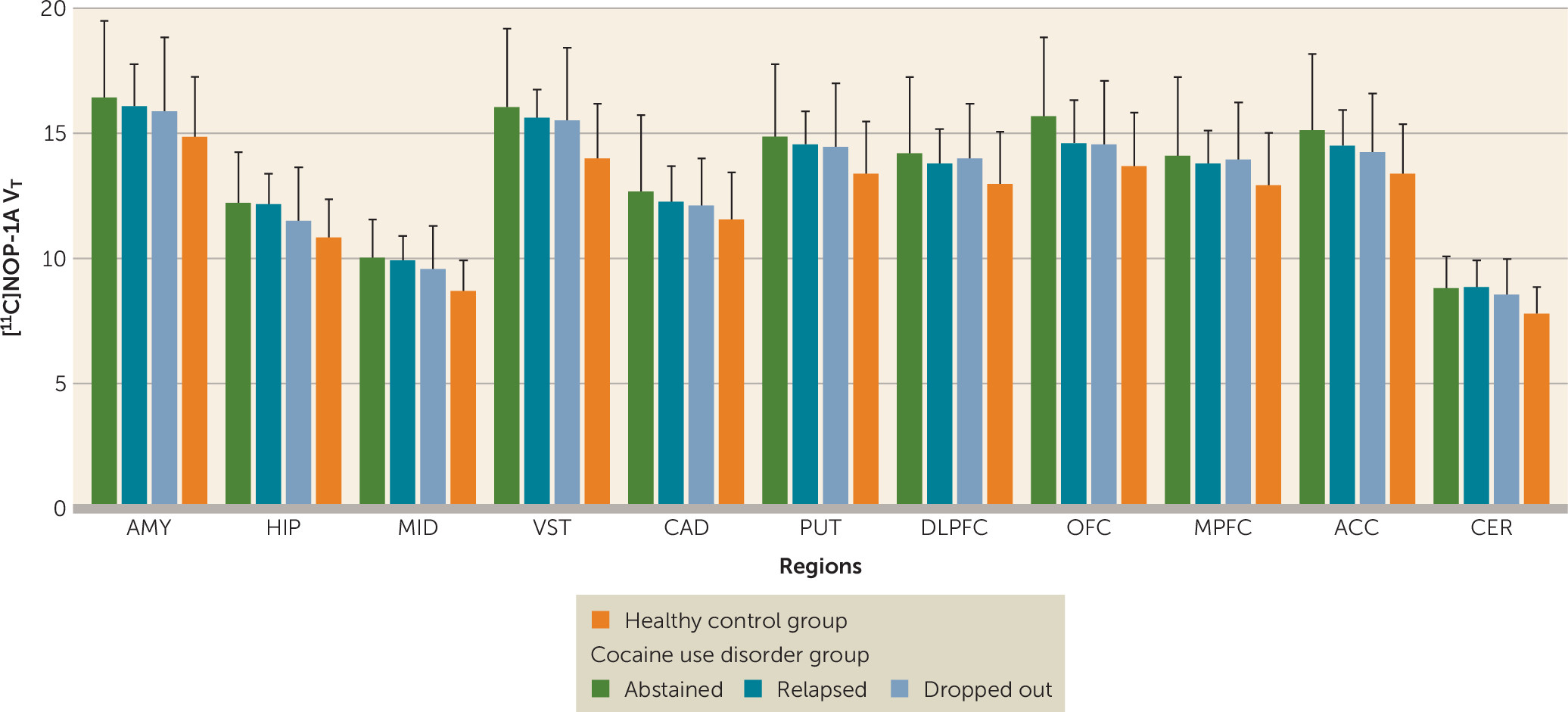
There were no significant differences in V
T between participants with cocaine use disorder who abstained compared with those who relapsed compared with those who dropped out during the follow-up period (linear mixed-model analysis: effect of final outcome status: F=0.13, df=2, 21, p=0.88; effect of region: F=230.65, df=10, 210, p<0.001; region-by-diagnosis interaction: F=0.49, df=20, 210, p=0.97) (
Figure 2). Stratification of the participants with cocaine use disorder on the basis of whether they relapsed or dropped out within the first 2 weeks (<2 weeks, N=9; >2 weeks, N=15) also revealed no group differences in [
11C]NOP-1A V
T (linear mixed-model analysis: effect of duration: F=1.24, df=1, 22, p=0.28; effect of region: F=223.81, df=10, 220, p<0.001; time-by-region interaction: F=0.65, df=10, 220, p=0.77). In addition, there were no significant correlations between V
T and the amount of voucher money earned during the follow-up period (midbrain, r=0.19, p=0.36; ventral striatum, r=0.18, p=0.41; and cerebellum, r=0.18, p=0.40). Lastly, V
T in the regions of interest was not predictive of time to relapse to cocaine use (midbrain, Exp [B]=1.0, p=0.99; ventral striatum, Exp [B]=0.97, p=0.78; and cerebellum, Exp [B]=1.02, p=0.95). These results were unchanged when the models were adjusted for potential confounding factors such as sex, Perceived Stress Scale ratings, and Cocaine Craving Questionnaire ratings.
Higher levels of perceived stress on the Perceived Stress Scale were predictive of less time to relapse (Exp [B]=1.08, p=0.05). No such relationship was observed between cocaine cravings (Cocaine Craving Questionnaire) and time to relapse (Exp [B]=1.00, p=0.88).
Relationships Between VT and Clinical Measures
No significant relationships were observed between regional [11C]NOP-1A VT and any of the other clinical measures in the cocaine use disorder group, including cocaine use frequency and duration, amount of money spent, perceived stress, anxiety, and cocaine craving.
Relationship Between VT and Age
In the healthy control group, there was a negative relationship between age and VT in the medial prefrontal cortex (r=–0.39, p=0.06) and dorsolateral prefrontal cortex (r=–0.36, p=0.08) but not in other regions. No such relationship between age and VT was observed in any of the regions in the cocaine use disorder group.
Discussion
In this [11C]NOP-1A PET study, we found that cocaine abuse in humans was associated with a generalized (approximately 10%) increase in binding to NOP in brain regions that mediate reward and stress behaviors (the four regions that were significant included the midbrain, ventral striatum, hippocampus, and cerebellum), that females have less NOP binding compared with males, and that increased stress during cocaine withdrawal predicts early relapse. An important negative result was the lack of a relationship between VT and relapse.
Increased [
11C]NOP-1A binding in cocaine users suggests a compensatory upregulation of NOP in response to decreased N/OFQ in the brain. This is supported by studies of rodents that were administered cocaine chronically, which showed decreases in N/OFQ in the nucleus accumbens, caudate-putamen, and substantia nigra/ventral tegmental area (
15,
16). However, NOP’s regulatory response to decreased N/OFQ in these rodents was shown to be variable at the level of the striatum (i.e., increased in the nucleus accumbens [ventral striatum]) and decreased in the caudate-putamen (
16). In the present study, PET data for the cocaine use disorder group, which showed an increase in NOP in the ventral striatum and putamen and no change in the caudate, was only partially consistent with these rodent data. The lack of an increase in NOP in the caudate, which has been observed in studies of both humans and rodents, is still noteworthy, because it may relate to the fact that the caudate is the only striatal subdivision in which dopamine transmission is not blunted in cocaine use disorder (
32). Studies examining the relationship between NOP V
T and amphetamine-induced dopamine release in people with cocaine use disorder might clarify this relationship. Behavioral paradigms that mimic chronic stress in rodents consistently show upregulation of NOP in the limbic brain regions (
33–
36). Thus, increased NOP in the cocaine use disorder group might also reflect an adaptive response to balance the effects of CRF, which increases stress and promotes relapse. Supportive of this hypothesis is a study in which an upregulation of NOP in the bed nucleus of the stria terminalis and the amygdala was observed following increases in CRF (
33,
37). Similar interactions between NOP and other stress-promoting neuropeptides, such as dynorphin, have also been described in rodents administered cocaine chronically (
16). Imaging studies with [
11C]NOP-1A investigating NOP-CRF and NOP-dynorphin interactions in cocaine use disorder should be considered, because such studies might clarify the interplay between stress and antistress neuropeptides in negative reinforcement and relapse. N/OFQ stimulates NOP to inhibit calcium channels and activate potassium channels (
38). This allows for N/OFQ to inhibit the release of multiple neurotransmitters, including dopamine, serotonin, acetylcholine, glutamate, and GABA (
39). Increased N/OFQ has been shown to inhibit cocaine-induced dopamine release in the nucleus accumbens (
12–
14). This mechanism has therapeutic potential, because it can decrease the cocaine reward experience. It is likely that N/OFQ directly modulates midbrain dopamine neurons to affect cocaine-induced dopamine release, because 50%−90% of tyrosine hydroxylase positive cells (TH+) in the ventral tegmental area and substantia nigra express NOP (
40,
41). However, an effect for presynaptic NOP on dopamine terminals cannot be ruled out, because administration of N/OFQ into the nucleus accumbens also blocks cocaine-induced dopamine release (
13). In addition, increased N/OFQ has been shown to decrease glutamate transmission in the reward- and stress-mediating brain regions, such as the cortex, midbrain, amygdala, and cerebellum (
42–
44). This mechanism also has therapeutic potential, because increased glutamate in cocaine use disorder has been linked to relapse and reinstatement (
45). These mechanistic investigations and the present PET study support clinical trials with a NOP agonist to increase N/OFQ transmission in cocaine use disorder. The inability to link NOP V
T with relapse in the present study cannot be viewed as less supportive of this conceptual model, because it is not possible to quantitate endogenous N/OFQ levels from a baseline [
11C]NOP-1A scan. [
11C]NOP-1A imaging paradigms that measure the regulatory response of NOP to stress/CRF might be more successful in predicting relapse in cocaine use disorder.
Sex-based differences have been reported for stress-mediating neuropeptides and their receptors, including CRF and kappa opioid receptors (
46,
47). To our knowledge, decreased NOP in females relative to males is the first report of sex-based differences in an antistress system in the brain. This unreported observation fell short of significance in a separate set of 15 healthy control subjects (females, N=5; males, N=10; 11 regions of interest; linear mixed-model analysis: effect of gender on V
T [p=0.055], region [p<0.001], gender-by-region interaction [p=0.012]) in our previous [
11C]NOP-1A alcohol study (
19). Studies to understand the clinical relevance of decreased NOP in females are necessary, because they might explain previously described behavioral and physiological phenomena, such as females who report greater subjective levels of stress showing increased sensitivity to CRF. Such studies may also provide clues as to the reasons why females are at higher risk for certain chronic stress disorders, such as depression, anxiety, and chronic pain.
Sex had no effect on the increased NOP in cocaine use disorder (i.e., there was no sex-by-diagnosis interaction). However, the effect size of the difference in [
11C]NOP-1A V
T in the cocaine use disorder group compared with the healthy control group was much larger in females compared with males (
Table 4). This is interesting, since rodent and human studies of chronic cocaine use have consistently reported more negative affect, withdrawal symptoms, and stress-induced relapse/reinstatement in females compared with males (
48). It is tempting to speculate greater decreases in resilience-mediating N/OFQ or increases in stress-mediating CRF (both of which have been shown to upregulate NOP receptors in basic investigations) as reasons for more unpleasant symptoms and subsequent relapse in females with chronic cocaine abuse. Future [
11C]NOP-1A PET studies should focus on these sex differences and also control for the fluctuations in sex hormones associated with menstrual cycle and hormonal contraceptive use.
The role of negative reinforcement in promoting stress and anxiety and leading to relapse to drug or alcohol use is well established in rodent models of addiction (
3). Consistent with this model, we found that increased stress during cocaine withdrawal is predictive of less time to relapse. However, we found no relationship between perceived stress and anxiety and the antistress/resilience conferring NOP (V
T). The study subjects were scanned after they abstained from cocaine for a minimum of 10 days, which may have limited the ability to detect such a relationship. PET studies focusing on the neurochemistry of withdrawal are warranted, because the behavioral symptoms experienced during this period predict relapse.
We were unable to exclude differences in [
11C]NOP-1A nonspecific binding (V
ND) and plasma free fraction (
fP) as contributors to V
T. Reassuringly, previous [
11C]NOP-1A-blocking studies in humans suggest that 50%−75% of V
T represents specific binding to NOP (
18). In summary, we demonstrated a significant increase in NOP in persons with cocaine use disorder. The data showed regionally selective increases in NOP that fell short of statistical significance and that were convincingly evidenced in the midbrain, ventral striatum, and cerebellum. Increased NOP in the cocaine use disorder group may be indicative of an adaptive response to decreased N/OFQ or increased CRF or both. The clinical development of NOP agonists to enhance N/OFQ transmission should be explored as a treatment for cocaine use disorder.