Obsessive-compulsive disorder (OCD) is a disabling condition with a lifetime prevalence of 2%−3% (
1), with only 40%−60% of patients achieving partial response to treatment (
2). Noninvasive brain stimulation may represent an alternative novel treatment, which can modulate neuronal excitability, activity, and plasticity (
3,
4). The cortical-striatal-thalamic-cortical (CSTC) loop circuit, which projects from the cortex to the striatum, from the striatum to the thalamus (via the globus pallidus), and then back to the cortex (
5,
6), has been implicated in OCD. The circuitry is commonly divided into three main loops: sensorimotor, cognitive, and limbic, involving the sensorimotor cortices, the dorsolateral prefrontal cortex, and the orbitofrontal and anterior cingulate cortices (OFC and ACC), respectively (
7). Substantial evidence suggests that OCD involves functional and anatomical abnormalities in the limbic CSTC loop, including the OFC, ACC, and ventral striatum (a portion of the striatum that receives projections from the OFC and ACC) (
5,
8).
These structures are often found to be hyperactivated in OCD patients during rest and further hyperactivated after symptom provocation, and their activity has been reported to be decreased after successful treatment (
9,
10). More specifically, the ACC has consistently been reported to be involved in processes that are impaired in OCD (
11), including integration of thought, motivation, and emotion with movement (
12), response selection before a movement occurs (
12), error monitoring (
13), and the detection of cognitive conflicts (
14). In recent years, several attempts to treat OCD with repetitive transcranial magnetic stimulation (rTMS) have been documented (
15), and a recent meta-analysis found active rTMS to be clinically and statistically superior to sham treatment (
16,
17). However, no consensus protocol has yet emerged with respect to stimulation target, frequency, and intensity (
16,
17).
H-coils allow deeper and broader penetration of electromagnetic stimulation into the brain. The H7 deep TMS (dTMS) coil was specifically designed to directly target the medial prefrontal cortex (mPFC) and ACC (
18). Indeed, a pilot study using the H7 coil found that high-frequency (but not low-frequency) stimulation over the mPFC and ACC regions significantly reduced OCD symptoms (
19). Here we report results of a prospective multicenter randomized double-blind study in which outcomes of dTMS targeting the mPFC and ACC were compared with those of sham stimulation. The primary outcome measure was the change in OCD symptoms as measured by the Yale-Brown Obsessive Compulsive Scale (YBOCS) (
20).
Methods
Study Design
The study was conducted at 11 sites—nine in the United States, one in Israel, and one in Canada—with active enrollment from October 2014 through February 2017. The study was approved by local institutional review boards and was registered at
ClinicalTrials.gov (NCT02229903). Considering the pilot study (
19), in which response to treatment did not reach a plateau after 5 weeks of treatments (daily treatment sessions 5 days a week, for a total of 25 sessions), we extended the treatment phase to 6 weeks, with one day for assessments (for a total of 29 treatment sessions). The study consisted of three phases: a 3-week screening phase, a 6-week treatment phase (consisting of 5 weeks of daily treatments 5 days a week and four treatments during the 6th week), and a 4-week follow-up phase.
Patients
A total of 100 patients were recruited through web advertisements (N=28) and referrals from local physicians (N=72), and the diagnosis of OCD as a primary disorder was confirmed by a certified clinician using the Structured Clinical Interview for DSM-IV. To be eligible, participants had to be between 22 and 68 years old, be receiving treatment in an outpatient setting, and have a YBOCS score ≥20. In addition, because we sought to recruit patients who had a limited response to previous treatments, patients had to be either in maintenance treatment with a therapeutic dosage of a serotonin reuptake inhibitor (SRI) for at least 2 months before randomization or, if they were not on an SRI, in maintenance treatment on cognitive-behavioral therapy (CBT) and have failed to respond adequately to at least one past trial of an SRI. SRIs and other antidepressants and D2 or D2/5-HT2 antagonist medications were allowed but could not be changed for at least 2 months before enrollment and throughout the study. (For full details on the patients’ baseline characteristics, see Tables S1 and S2 in the online supplement.) The main exclusion criteria were any primary axis I diagnosis other than OCD, severe neurological impairment, and any condition associated with an increased risk of seizures. All patients provided written informed consent after receiving a complete description of the study.
Participants who met the eligibility criteria were allocated in a 1:1 ratio to one of the two treatment groups—sham or active dTMS—through a stratified randomization scheme using the random number generator in SAS, version 9.4. A central interactive web-based randomization system developed for the study was used to assign to each participant a unique randomization code, which determined the participant’s assignment. The unique randomization code matched one of the preprogrammed treatment cards maintained at the clinical sites.
Assessments
Clinical severity rating scales included the 10-item YBOCS, the 21-item Hamilton Depression Rating Scale (HAM-D) (
21), the Sheehan Disability Scale (
22), and the Clinical Global Impressions severity scale (CGI-S) and a modified version of the improvement scale (CGI-I) (
23) (see the
online supplement). Safety evaluations included monitoring of adverse events, assessment of vital signs, physical and neurological examinations, urine pregnancy tests, and the Scale for Suicide Ideation (
24). All raters underwent a uniform training program and certification for the administration of the rating scales.
Personalized Symptom Provocation
Following previous studies (
19,
25–
27), a 3–5 minute individualized symptom provocation was performed before each treatment to activate the relevant neuronal circuit. A hierarchically ordered list (from less to more distress provoking) of personalized obsessive-compulsive symptom provocations was designed by a clinician (a psychologist or psychiatrist) together with the patient during the first assessment meeting. All provocations, from all the sites, were reviewed and approved by a central OCD expert psychologist (L.C.) and were recorded on the case report forms. The provocation procedure was administrated by a certified and trained staff member before each treatment session, with the aim of achieving a self-reported distress score between 4 and 7 on a visual analogue scale ranging from 1 to 10. The staff guided the patient through the hierarchical list and chose the item that triggered the required distress score. Once the score was achieved, the patient was asked to keep thinking about this specific obsession during the treatment. To maintain the obsessive-compulsive arousal, the patient was reminded once, 3–5 minutes after the start of the treatment, to continue thinking about the provocation (e.g., “Please keep thinking about the dirty handle”).
dTMS Intervention
dTMS was administered using a Magstim Rapid2 TMS stimulator (Magstim, Whitland, U.K.) equipped with a unique H-shaped coil design (
18,
28,
29). The H-coil version used in this study was the H7 (Brainsway, Jerusalem, Israel). When placed 4 cm anterior to the foot motor cortex and used at 100% of the leg resting motor threshold (RMT), the H7 coil stimulates the dorsal mPFC and ACC bilaterally. This has been shown in computer simulations (
30) and in electric field measurements in a head model filled with saline solution (
31). Approximately 70 cm
3 of the neuronal volume is stimulated above the neuronal activation threshold (100 V/m) (
31). A map of the H7 coil field distribution is shown in Figure S1 in the
online supplement.
During the RMT procedure, the optimal spot on the scalp for stimulation of the motor cortex was localized and a resting motor threshold was defined. A participant’s RMT was determined before the first treatment and at the beginning of each week by ascertaining the coil position that elicited the minimal involuntary contractions of the feet (three of six attempts). The active treatment group received 20 Hz dTMS at 100% of RMT, with 2-second pulse trains and 20-second intertrain intervals, for a total of 50 trains and 2,000 pulses per session. The sham treatment group received treatment with identical technical parameters, which induced scalp sensations but without penetration of the electric field into the brain, as previously described (
32).
Blinding
Sham treatment produces a scalp sensation, and participants were told that face or hand twitching might occur during either (active or sham) treatment. Patients, operators, and raters were blind to treatment condition. Each patient was assigned a magnetic card that determined the coil in the helmet that would be activated after placement of the helmet over the mPFC-ACC location (
25,
26), and raters were not present while treatments were administered. After the first treatment, patients were asked which treatment they thought they were assigned to (active or sham) and to choose one of the following answers: “Strong belief that I received active treatment”; “Moderate belief that I received active treatment”; “I do not know”; “Moderate belief that I received sham treatment”; “Strong belief that I received sham treatment.”
Primary and Secondary Efficacy Objectives
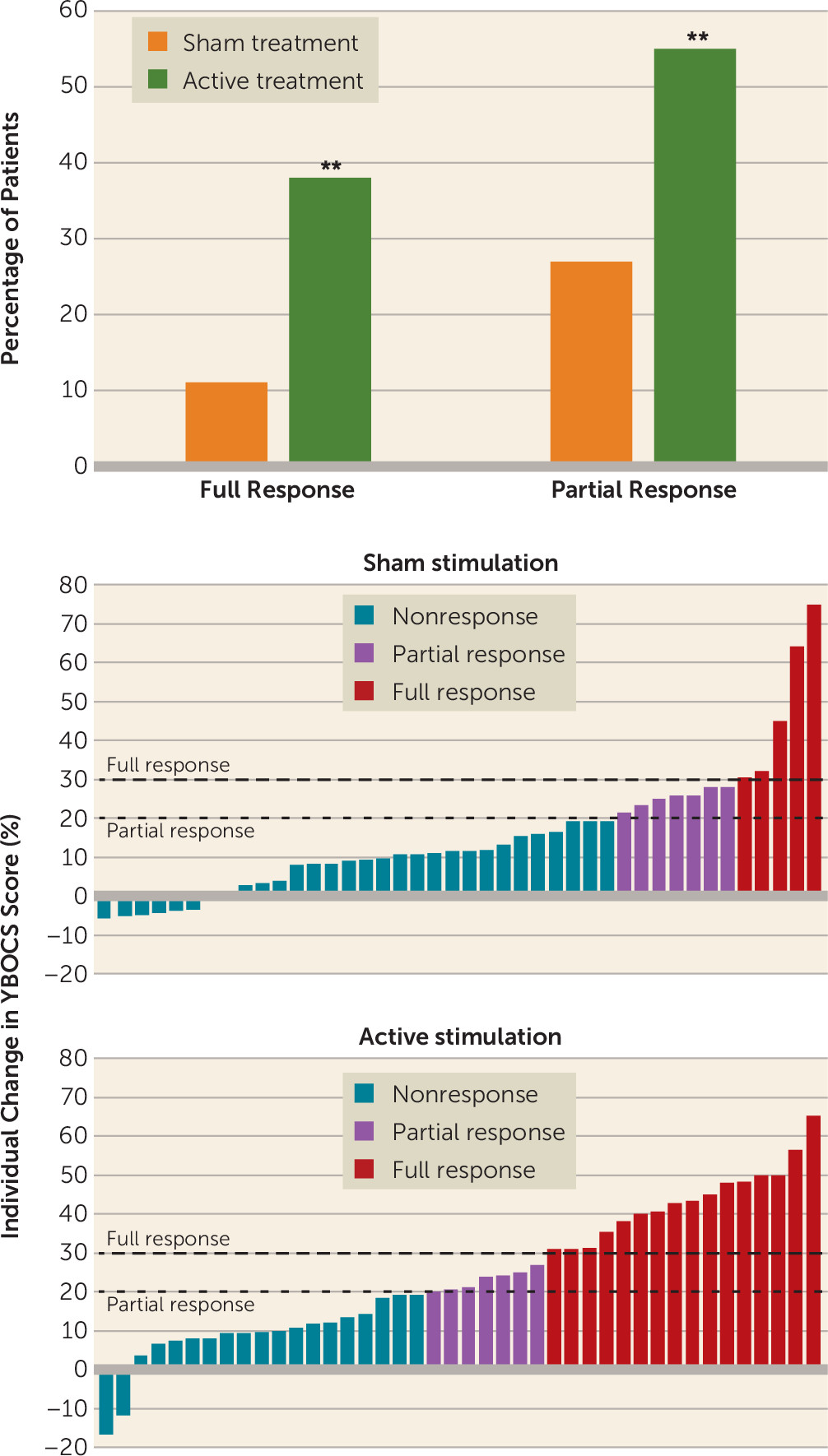
The primary outcome measure was the change in YBOCS score from the baseline assessment to the posttreatment assessment. The secondary outcome measures were the change in YBOCS score from the baseline assessment to the 1-month follow-up assessment and rate of full response. Because we recruited patients with inadequate response to previous treatments, a full response was defined as a reduction of ≥30% and a partial response as a reduction ≥20% in YBOCS score (
33) from the baseline assessment to the posttreatment assessment. Additional secondary outcome measures were change from baseline to posttreatment assessment in CGI-I, CGI-S, and Sheehan Disability Scale scores. Exploratory outcome measures included change in HAM-D score from the baseline assessment to the posttreatment assessment and from the baseline assessment to the follow-up assessment.
Sample Size and Power Analysis
In our pilot study, the mean reduction in YBOCS score from baseline to week 5 was 6.7 points (SD=3.86) in the active treatment group and 1.0 point (SD=2.88) in the control group. A more conservative difference of 3 points between groups was used to calculate the sample size, assuming a standard deviation of 4.0 points. The analysis revealed that a total of 78 participants (39 per group) would provide a power of approximately 90% at a 5% significance level. The minimum sample size was increased by 20%, to 49 participants per group (for a total of 98 participants), to account for potential study dropout.
Statistical Analysis
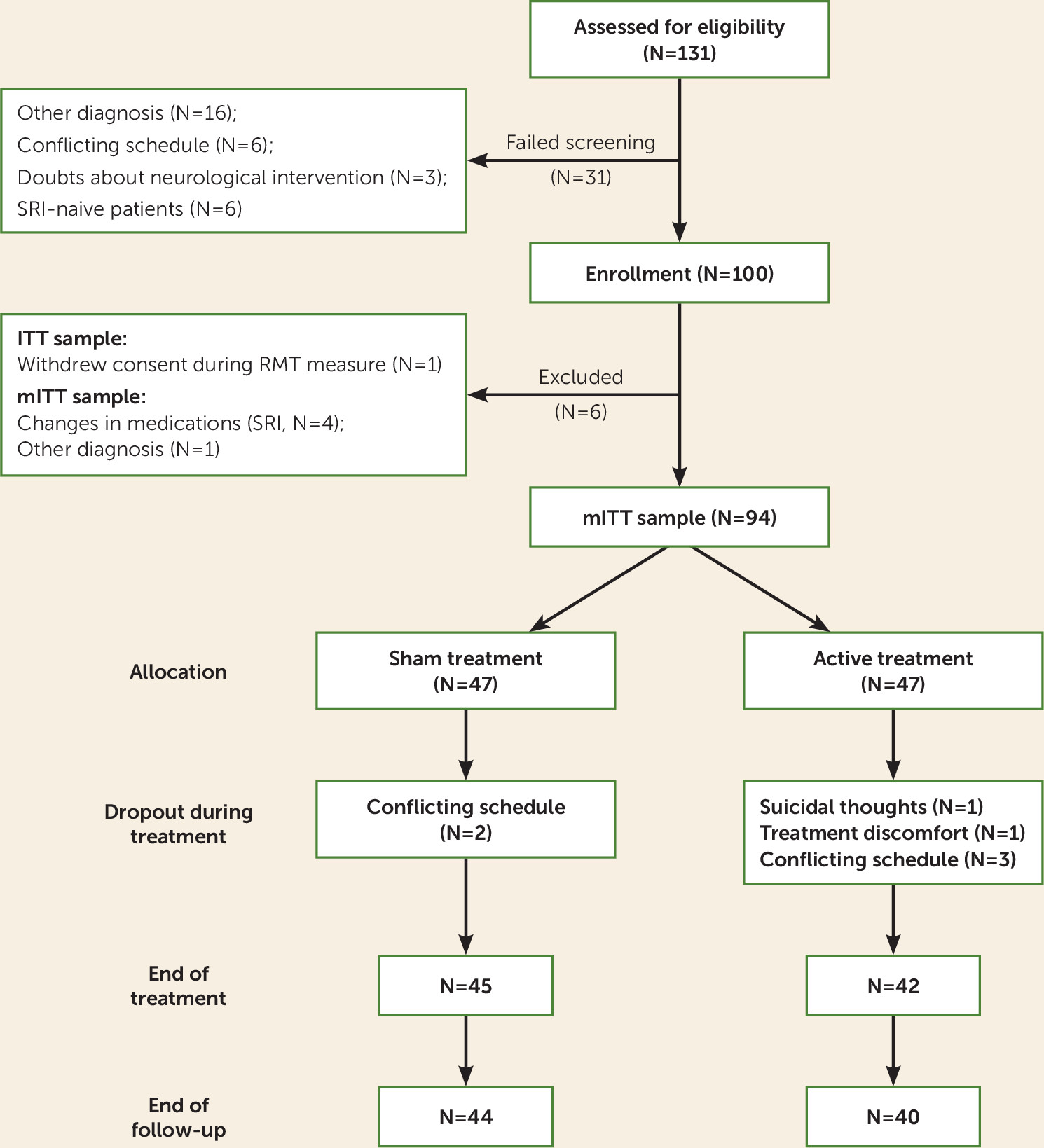
The intent-to-treat (ITT) analysis set includes all patients who underwent randomization and received at least one active or sham treatment (even if found to be enrolled in error during the study; see Table S2 in the online supplement). The modified ITT analysis set was defined in the protocol as the main analysis set for primary and secondary results. It includes all patients who met the study eligibility criteria, underwent randomization, and received at least one active or sham treatment. The decision to exclude five patients from the full (ITT) sample (i.e., the determination of the modified ITT analysis set) was taken by the principal investigator before the blind was broken or any analysis had begun (for exclusion criteria and ITT results, see Table S2 in the online supplement).
Statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, N.C.). The principal statistical analysis was performed using a repeated-measures analysis of covariance approach (SAS MIXED procedure). It consisted of a comparison between the treatment groups’ YBOCS score slopes of change, derived from the time-by-treatment interaction term from the repeated-measures model. The analysis aimed to compare the YBOCS slopes of change from baseline between study arms, including the following fixed effects: time from randomization, treatment group, time-by-treatment interaction, use of SRIs and any other medications, psychotherapeutic behavioral interventions at enrollment, and baseline YBOCS score. The individual subject intercept and the time effects were also included in the model as random effects (random intercept and slope model). The adjusted mean changes in YBOCS score from baseline to posttreatment assessment were estimated from the model (least square means) for both groups, as well as the difference between the adjusted means, and are presented together with 95% confidence intervals. Binary efficacy and other categorical measures were compared between the study groups at the posttreatment and follow-up assessments with the chi-square test or Fisher’s exact test. For missing data analysis, please see the online supplement.
Results
A total of 100 patients with OCD were enrolled in the study (demographic characteristics and data on dropouts of the ITT analysis are presented in Table S1 in the
online supplement). The ITT sample included 99 patients and the modified ITT sample included 94 patients (
Figure 1).
Baseline Assessment Scale Values
Table 1 presents the distribution of baseline values for all assessment scale data represented in the efficacy analyses. No statistically significant differences were found between the study groups with respect to the baseline efficacy assessment scale data, or with respect to the primary, secondary, and exploratory efficacy endpoint variables. The mean baseline HAM-D score was 10.1 (SD=5.74) for the active treatment group and 10.7 (SD=5.47) for the sham treatment group, indicating that most patients were not depressed.
Primary Efficacy Analysis
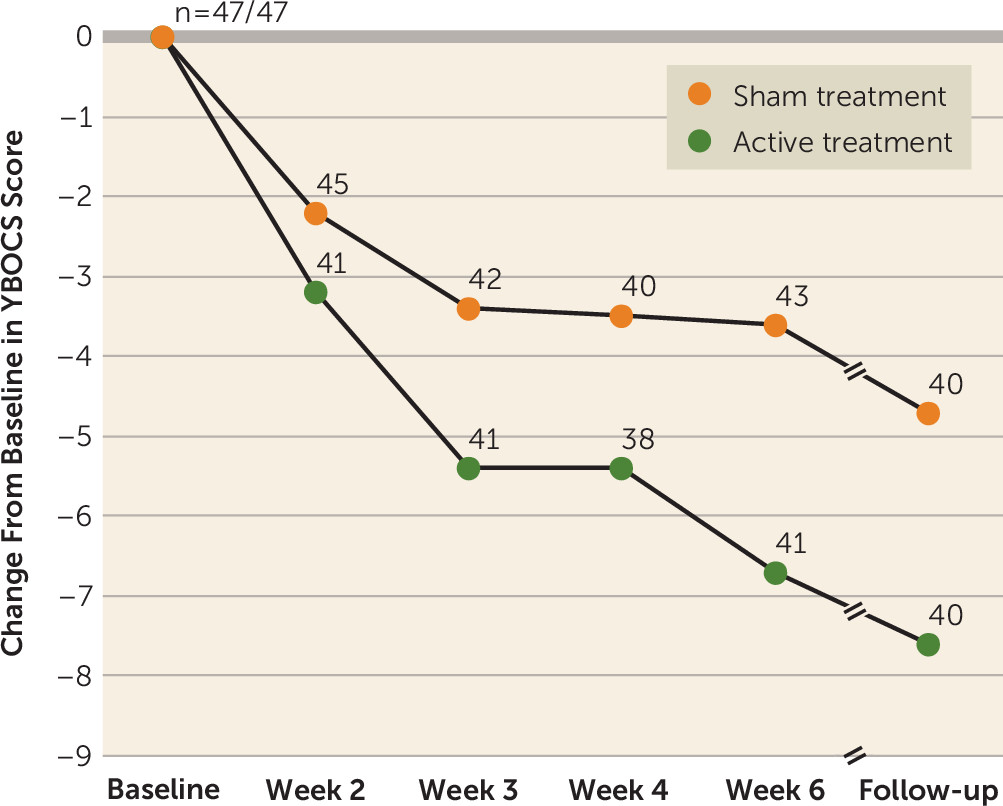
At the posttreatment assessment at 6 weeks, the YBOCS score decreased significantly from baseline in both the active (−6.0 points, 95% CI=4.0, 8.1) and sham (−3.3 points, 95% CI=1.2, 5.3) treatment groups (estimated slopes). The difference in slopes of change in YBOCS score between the two groups was statistically significant at the posttreatment assessment (2.8 points, p=0.01), for an effect size of 0.69.
Secondary Efficacy Analysis
The effect was also present at the 4-week posttreatment follow-up assessment, at which point the mean YBOCS score had decreased by 6.5 points (95% CI=4.3, 8.7) in the active treatment group and by 4.1 points (95% CI=1.9, 6.2) in the sham treatment group (p=0.03), for an effect size of 0.62. The rate of full response (a reduction ≥30% in YBOCS score) at the posttreatment assessment in the active treatment group was 38.1% (16/42), compared with 11.1% (5/45) in the sham treatment group (p=0.003) (
Figure 3).
The rate of full response at the follow-up assessment was 45.2% (19/42) in the active treatment group, compared with 17.8% (8/45) in the sham treatment group (p=0.006, chi-square test). The rate of partial response at the follow-up assessment was 59.5% (25/42) in the active treatment group, compared with 42.2% (19/45) in the sham treatment group (p=0.106, chi-square test).
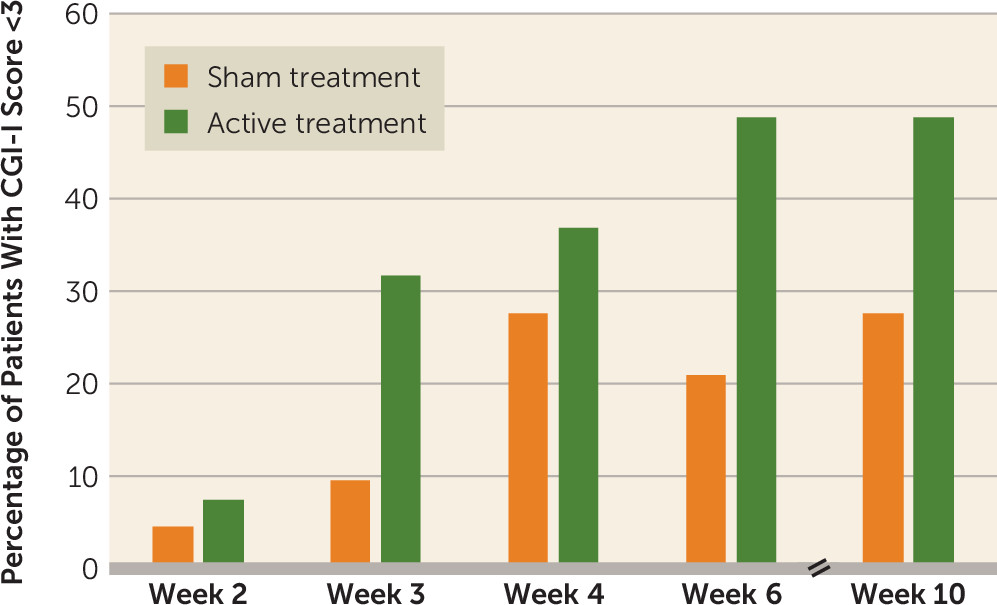
The CGI-I scores were classified into two categories: improved (moderately improved to very much improved) and not improved (minimally improved to very much worse). The CGI-I categorical analyses demonstrated a significant difference between the active and sham treatment groups at the posttreatment assessment. In the active treatment group, 49% of participants (20/41) reported feeling moderate to “very much” clinical improvement, as compared with only 21% (9/43) of participants in the sham treatment group (p=0.011) (
Figure 4).
The change from baseline in CGI-S scores was classified into three categories: improved (e.g., changed from moderately ill to mildly ill), no change, and worsened (e.g., changed from mildly ill to moderately ill). The CGI-S score was also found to be statistically significant at the posttreatment assessment, with higher rates of patients rated improved in the active treatment group as compared with the sham treatment group (61% [25/41] and 32.6% [14/43], respectively; p=0.022). The positive CGI-I and CGI-S results were also observed at the follow-up assessment (CGI-I: 49% [19/39] compared with 27.5% [11/40]; CGI-S: 64% [25/39] compared with 45% [18/40]), although the differences were not statistically significant.
The mean Sheehan Disability Scale score decreased by 3.8 points (95% CI=1.5, 6.1) in the active treatment group and by 3.0 points (95% CI=0.8, 5.3) in the sham treatment group from baseline to posttreatment assessment, with no statistically significant difference between the groups.
Exploratory Analysis
The majority of patients did not suffer from comorbid depression (the mean HAM-D scores at baseline were 10.0 and 10.9 for the two treatment groups), and in both groups HAM-D score decreased by 2.1 points from baseline to posttreatment assessment.
Blinding Assessment
The most frequent answer participants in both groups gave when asked to guess which treatment they were assigned to (and given the choice of “moderate” or “strong” belief that they had received active or sham treatment or “do not know”) was that they did not know (44% in the active treatment group and 47% in the sham treatment group). Forty-four percent of the active treatment group and 31% of the sham treatment group correctly guessed (moderate or strong belief) their assigned treatment, and 12% in the active treatment group and 22% in the sham treatment group incorrectly guessed (moderate or strong belief) their assigned treatment. Thus, around two-thirds of the study’s participants (66% of the active treatment group and 69% of the sham treatment group) were not aware of or incorrectly guessed the type of treatment they received. In addition, the answer to the treatment type question was not found to correlate with the actual treatment that was received (p=0.104).
Adverse Events and Dropouts
Thirty-five participants (73%) in the active treatment group and 35 (69%) in the sham treatment group reported adverse events, with no statistically significant difference between groups (p=0.639, chi-square test). The adverse events reported are typical of those reported in TMS studies, the most frequent being headache (37.5% of participants who received active treatment and 35.3% of those who received sham treatment); the difference between the groups was not statistically significant.
One serious adverse event was reported in the study. After receiving two treatments, one participant in the active treatment group reported having significant suicidal thoughts, which had preceded the start of the treatment sessions, although the patient had not mentioned this symptom earlier. The investigator and the participant decided that hospital admission would be appropriate. The participant reported that his suicidal thoughts were related to escalating problems with his family and not to the study treatments.
The dropout rate was about 12% for both groups (active treatment group, 6/48 patients; sham treatment group, 6/51 patients), with no significant difference between the groups.
Discussion
This is the first study of dTMS to explore the safety, tolerability, and efficacy of dTMS in OCD. The results indicate that dTMS stimulation over the mPFC and ACC is a safe and effective intervention for improving OCD symptoms in patients who failed to receive sufficient benefit from treatments with SRIs and CBT. The reduction in YBOCS score was significantly greater at the posttreatment assessment and remained significant at the 1-month follow-up assessment in the active as compared with the sham treatment group. In addition, the positive effect of the treatment was evident as a higher response rate and better CGI-I and CGI-S results after active as compared with sham treatment. The response rate in the sham treatment group was low and in agreement with previous sham-controlled TMS studies (
15). The high-frequency dTMS using the H7 coil was well tolerated by patients. No severe adverse events such as seizures occurred, and the most frequent side effects included mild headache during or immediately after stimulation, a pattern that is consistent with a recent comprehensive review (
34).
As an extension of our pilot study (
19), this study is the first to stimulate the ACC and the mPFC components of the CSTC in a multicenter study. Although the superiority of active rTMS treatment compared with sham treatment in OCD has been demonstrated in several studies, an accepted treatment protocol has not been adopted and different stimulation sites have yielded contradictory findings (
2). Specifically, stimulation of the dorsolateral prefrontal cortex has demonstrated inconsistent results, and stimulation of the supplementary motor area and the pre-supplementary motor area (despite not being an inherent part of the CSTC implicated in OCD) and the OFC have yielded more positive results (
35). As for OFC stimulation, to the best of our knowledge, only one relevant placebo-controlled (
36) and one crossover pilot study (
37) have been published (they yielded positive results that were significant and fell short of significance, respectively). Thus, the ACC stimulation pilot study (
19) and the present study are the first to show consistent positive results from stimulation of a central part of the CSTC.
In a recent meta-analysis based on 17 studies (
38), SRIs were found to be superior to placebo in OCD, with an average 3.2-point change in the weighted mean difference in YBOCS score over 10–13 weeks. However, our study showed the same effect (a difference of 2.8 points in YBOCS score between the active and sham treatment groups) in a shorter time (6 weeks). Furthermore, the patients recruited to the present study had experienced inadequate response to SRIs and CBT, which implies that dTMS and SRIs exert their effects on different neuronal mechanisms.
Our study also included integration of tailored obsessive-compulsive exposures as part of the treatment. Based on previous studies (
19,
25–
27), we hypothesized that activation of the relevant circuitry may increase the therapeutic effect of the dTMS. Accordingly, personalized obsessive-compulsive symptom provocation exposures were used at the beginning of each treatment session to activate the pathological circuitry. Although this may be considered brief exposure therapy, the fact that both groups underwent the same exposure procedure suggests that the observed clinical effects were due only to stimulation.
Limitations, Implications, and Further Questions
There are some limitations to this study. First, the effect of provocation was not controlled, and the relevant brain activity was not recorded; hence, the exact contribution of the exposure procedure is not fully known. Second, the extent to which the ACC and the mPFC were stimulated needs to be further investigated in functional brain imaging studies. And third, although the patients were asked about their past treatment history, this was not validated with source documentation such as records of filled prescriptions or other objective information.
The intriguing finding of an additional benefit for OCD patients who did not respond adequately to pharmacological or psychological treatment suggests that dTMS may involve a different mechanism. Accordingly, we recommend considering the option of adding dTMS to treatment when the response to a proper psychological or pharmacological intervention is inadequate. (This recommendation takes into consideration that the benefit to risk ratio of this treatment is favorable.)
It would be optimal if clinicians could predict which patients are likely to respond to treatment. For example, in the pilot study (
19) the amplitude of the theta frequency band (4–8 Hz) in response to a Stroop task correlated with the amplitude of the change in YBOCS score. The possibility of corroborating such measures at baseline, after a validation study in a large sample of patients, could help predict the response and selection of the appropriate population for this 6-week treatment course. Further refinements of the stimulation and provocation parameters, treatment during a maintenance phase, and the combination of dTMS treatment with CBT should be investigated. Studies that combine a precise behavioral challenge with neuromodulation and neuroimaging and that attempt to identify potential responders should also be considered.
Acknowledgments
The authors thank Dr. Jon Grant as well as Amit Gino and Shlomi Fishman for their important continuous participation in this study.