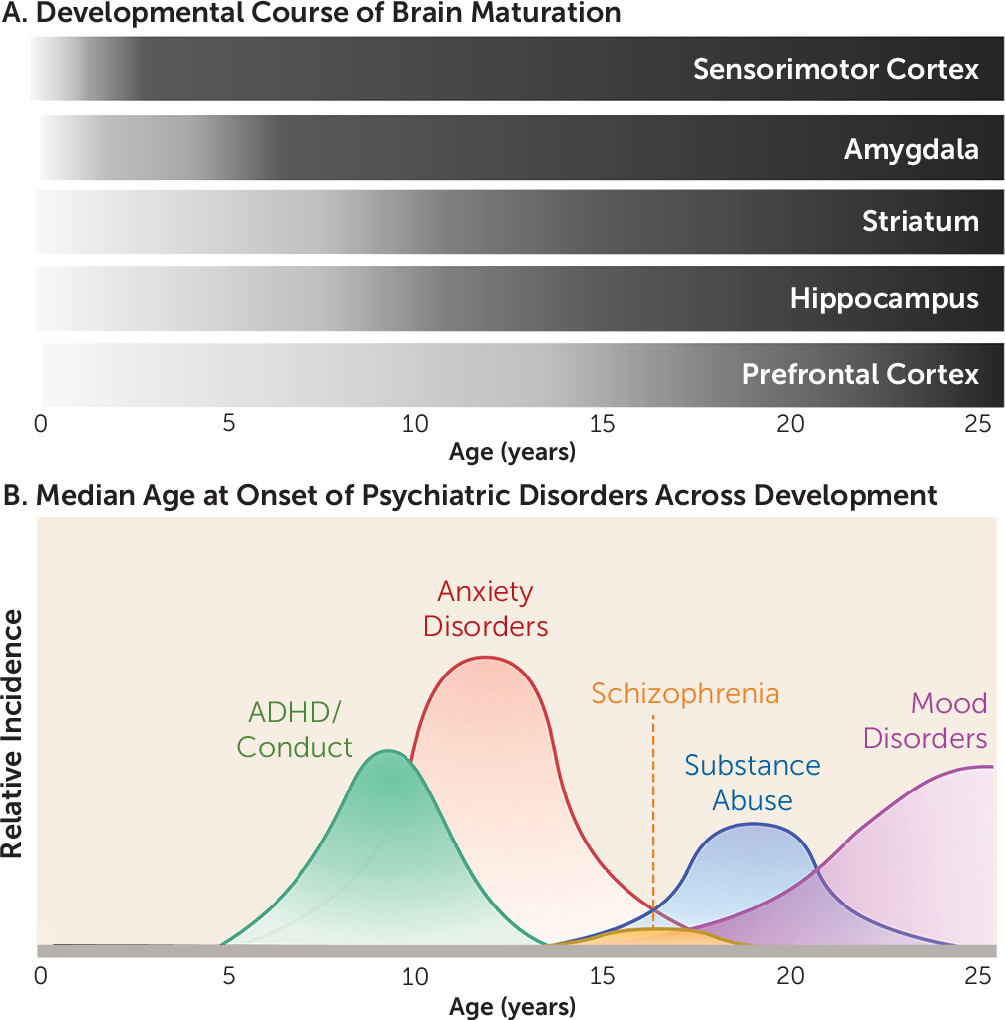
In recent years, significant interest has been directed to understanding the interplay between the specific neurobiological and behavioral factors that characterize developmental stages. Research in this area has burgeoned in large part as a result of the motivation to identify why particular individuals are susceptible to negative outcomes. Indeed, as illustrated in
Figure 1, up to three-quarters of all psychiatric disorders emerge before age 24 (
1–
3). Moreover, the developmental emergence of psychopathology has been associated with greater severity of symptoms, comorbidities, and higher recurrence rates (
4–
6). More often than not, cognitive, emotional, and behavioral responses are based on prior experiences that can begin in the earliest stages of life. Thus, a complete understanding of cognitive and emotional functioning must include a deep appreciation of early-life experiences and environments (
7). To gain insight into how developing systems function normally, as well as how they may go awry in psychiatric disorders, it is important to delineate how complex developmental trajectories and external factors interact to influence neural circuitry. Neurobiological research has consistently illustrated high conservation in motivated learning and emotion-related systems across species, lending support to the translational value of preclinical animal models (
7,
8).
Across species, the developing brain is characterized by a high degree of neural plasticity, which is ecologically advantageous, as it allows for the refinement of neurocircuitry that is specifically tuned to the demands of the surrounding environment. However, the same capacity for neural alteration can make the developing brain particularly vulnerable. Indeed, perturbations to maturing systems can disrupt the refinement of cortical circuits, resulting in long-term consequences for both cognitive and emotional functioning. Moreover, developmental dysregulation of emotional memory systems is a principal component of many psychiatric disorders.
Infantile And Childhood Development Of Fear And Threat Responding
One of the earliest examples of functional neurocircuitry commonly studied is the circuitry underlying threat responding. From a very young age, fear and anxiety-related responding can be advantageous. However, numerous psychiatric conditions in which altered fear processing is excessive also emerge during development as the brain is undergoing complex and dynamic changes. Thus, understanding the development of fear and anxiety systems is critical for developing strategies to mitigate fear, stress, and anxiety disorders.
The processing and responding to threats in early life has been shown to differ fundamentally from that observed in adulthood (
9–
11). In rodents, fear memories acquired before postnatal day 10, during a highly caregiver dependent developmental window of rodent infancy before brief excursions from the nest begin to take place (similar to the first year of human infancy [
7]), are not as robust or persistent as those acquired later in life and remain susceptible to forgetting through a process known as infantile amnesia (
12–
15). Functional emergence of the amygdala during childhood (
Figure 1) (and following postnatal day 10 in rodents) coincides with more traditional fear learning to conditioned stimuli (
11,
16–
18), although retention of fear-related memories is shorter compared with adults (
15). Moreover, learned fear associations are not subject to forms of contextually mediated relapse (e.g., renewal, reinstatement, and spontaneous recovery) after extinction (
19,
20), which are commonly observed in adults and have been taken to indicate that extinction does not
erase a fear memory, because conditioned responding can return after a change in context, reexposure to an aversive outcome, or the passage of time (
21). In addition, relative to cued conditioning, contextual conditioning in rodents emerges later in development (
22–
25).
As additional regions known to be crucial for fear learning are engaged across development, adult-like fear patterns emerge. In particular, circuitry appears to be largely dependent on the amygdala in early life, with the network increasing in complexity by integrating prefrontal and hippocampal connections as these regions and the connections between them develop over the course of childhood. For example, the inverse activity with the amygdala of the prelimbic prefrontal cortex (PFC) for fear expression and the infralimbic PFC for fear attenuation that is observed in adults (
26,
27) emerges after the juvenile period (
19). This corresponds to the longer-lasting retention of cued fear associations. In addition, both contextual fear memory and contextually mediated relapse emerge with the maturation and integration of hippocampal circuitry (
28) (
Figure 1).
Impact Of Stress On Infantile And Childhood Development Of Fear, Anxiety, And Threat Responding
Interestingly, mounting evidence suggests that exposure to stressors during early life can shift the timing of prefrontal and subcortical development (
29–
33). Because of the high dependence developing individuals have on their caregiver, it is not surprising that deviations in caregiving, including physical and emotional abuse, neglect, parental death or incarceration, and child institutionalization, have been a point of focus for their influence on the development of circuitry underlying emotion and motivated behavior. Although caregivers play a central role in suppressing threat reactivity during infancy and early childhood in rodents, nonhuman primates, and humans (
34–
37), disruptions to this role can contribute to differential development of fear systems that may increase the propensity for later psychopathology (
7,
38–
40).
A compelling series of studies has led to the suggestion that early-life stress may actually initiate precocious structural and connectivity profiles of the fear neurocircuitry (
29,
30,
41,
42) that have been associated with adult-like patterns of behavior (
41,
43). While these changes may initially be adaptive for meeting the needs of the developing organism in an adverse environment (
30,
44), long-term consequences may also arise from altered developmental trajectories. Indeed, changes to the brain following early-life stress have been associated with psychopathology, including symptoms of depression and anxiety as well as substance use disorders (
5,
29,
45–
47).
Adolescent Development Of Motivated Responding
While robust structural changes occur within specific brain regions during infancy and childhood, the refinement of connectivity within and between brain regions has been shown to play a central role in adolescent development (
48,
49). Notably, a shift from predominant connectivity between anatomically proximal regions to functional interconnectivity, especially between distributed networks, increases as the adolescent period progresses (
50,
51), and this has been correlated with greater efficiency of cortical processing (
52). Moreover, heightened plasticity and the formation of integrated circuitry allows adolescents to interpret the demands of complex and variable environments and responding accordingly, making the adolescent brain well suited to forms of learning that occur in uncertain or changing environments as the individual establishes an independent life (
53–
56).
At the same time, adolescence reflects a “sensitive window” during which circuit-level formation is highly responsive to environmental information. For example, the characteristic features of the adolescent brain, such as a predominance of subcortical regions over the prefrontal cortex, promote heightened sensitivity to both appetitive and aversive emotionally salient cues. Moreover, states of heightened emotional arousal disrupt deliberative executive functioning and inhibitory control to a greater extent during adolescence than in other age period (
57–
61). Although the capacity for encoding an appetitive memory is apparent from early in infancy (
62), interactions between reward circuitry and behavior have been a preeminent focus of research during later childhood and adolescence, because of an apparent hypersensitivity to reinforcers (
61,
63,
64) and even cues
signaling a potential reinforcer (
55,
57). Sensitivity to threat is also a marked characteristic of adolescence. Indeed, recent evidence from both human and animal studies has indicated that adolescents exhibit an increased acquisition of threat responding (
65,
66) as well as diminished extinction learning and extended fear retention relative to younger and older individuals (
67–
71). This is particularly interesting given that it occurs despite apparent adult-like patterns of fear responding in the juvenile period immediately preceding adolescence (
23,
67).
Peaks in sensation seeking during adolescence are often attributed to immaturities in frontostriatal circuitry (
72,
73), with improvements in cognitive control coinciding with continued development of this circuitry across the adolescent period (
74). On the other hand, substantial changes in the reactivity and connectivity of the amygdala, hippocampus, and PFC are believed to mediate altered fear learning during this time (
23,
67,
75–
77). Indeed, development of the PFC is protracted relative to most other brain regions, continuing well into adolescence (
78–
80). Meanwhile, the earlier maturation of subcortical limbic regions (e.g., the nucleus accumbens and amygdala) can result in disproportionately higher activity emerging in subcortical regions relative to the PFC during adolescence (
64) (
Figure 1). Notably, experimentally inducing a functional imbalance of this kind between the late-developing PFC and earlier-developing subcortical limbic regions has been shown to disrupt performance in an inhibitory learning task (
81), indicating that this characteristic of adolescent brain development may be a critical determinant of the capacity for behavioral regulation (
82).
With regard to fear neurocircuitry, amygdala projections to the cortex undergo significant development during adolescence (
83–
85), with bidirectional prelimbic-amygdala synapses maturing earlier than infralimbic-amygdala synapses (
86). In addition, during early adolescence, connectivity between basolateral amygdala and prelimbic cortex as well as ventral hippocampus and prelimbic cortex actually appears to be increased (
75). Connectivity between prelimbic cortex and amygdala has been linked to the expression of fear, while connectivity between infralimbic cortex and amygdala is critical for attenuating fear (
87,
88). Thus, the protracted development of the latter circuit, combined with an overall increase in amygdala signaling, may explain a range of adolescent behaviors indicative of threat sensitivity. In addition, an inability to retrieve contextual fear memories has been observed specifically during adolescence and has been linked to a lack of memory retrieval–associated signaling in the hippocampus (
23).
Similarly, in humans, both functional connectivity and the degree to which prefrontal activity synchronizes with regions such as the amygdala or hippocampus increase from childhood to adulthood (
76,
89,
90). Moreover, connectivity between amygdala and PFC appears to become more negative with age, reflective of an inverse correlation (and perhaps top-down inhibition) between activity in these regions (
76).
Impact Of Stress On Adolescent Development Of Motivated Responding
As with earlier developmental stages, the extensive changes in neural circuitry occurring during adolescence leave the brain susceptible to environmental stressors that may alter normative developmental trajectories. In particular, because of the social restructuring that characterizes the transition to adolescence, in which there is a shift in focus from relationships with family members to relationships with peers (
91,
92), exposure to social stressors can profoundly affect the normative developmental trajectory of the brain (
5,
93–
95). Notably, the effects of stress appear to be most robust when the stress occurs during adolescence (
5,
96,
97) and can include a reduced ability to regulate distress (
95,
98–
100) and a marked increase in susceptibility to psychopathology (
101). Exposure to social stress is just one example of the impacts that the environment can have on brain and behavioral development. Additional factors, such as diet, exercise, and drug use, among many others, have also been shown to dramatically influence brain development and contribute to the prevalence of psychiatric disorders.
Neurodevelopmental Studies In Psychiatric Populations
In addition to preclinical studies and neuroimaging studies of normative human brain circuit development, there have recently been parallel efforts to identify brain circuit alterations in psychiatric disorders that frequently emerge in developing populations, based on the assumption that there are discrete neural circuit alterations that correspond to the early signs and symptoms characterizing these disorders. The articles by Tromp et al. (
102) and Jalbrzikowski et al. (
103) in this issue of the
Journal highlight these latest efforts.
With regard to anxiety disorders, which are among the most common psychiatric illnesses in adolescents, affecting as many as 1 in 10 (
1,
104), there have been extensive investigations, using a variety of neuroimaging modalities, including task-based functional MRI (fMRI) studies, diffusion tensor imaging (DTI), and resting-state fMRI (rsfMRI) analyses. A general consensus among the task-based fMRI studies has been that there is marked elevation of amygdala activity in patients with anxiety disorders (
105). In addition, decreased resting-state connectivity has been identified in patients with anxiety disorders compared with control subjects between the amygdala nuclei and prefrontal cortical structures, including the anterior cingulate cortex, medial PFC, and orbitofrontal cortex (
106–
108). Structural connectivity studies using DTI in patients with anxiety disorders have also identified decreased functional anisotropy, a measure of white matter microstructure, in the uncinate fasciculus, a white matter tract connecting cortical and hippocampal regions with subcortical structures, including the amygdala (
109,
110). However, most of these studies were performed in adult patients with anxiety disorders. With regard to hyperactivation of the amygdala, the few studies in children and adolescents with anxiety disorders are consistent with studies in adult patients (
111,
112). In addition, studies in young rhesus monkeys with increased anxiety-related behavioral temperament have reported decreased functional connectivity between the dorsolateral PFC and central amygdala as well as heightened metabolism in the amygdala (
108,
113).
These findings should be considered in the context of the normative developmental changes in PFC-amygdala connectivity. As outlined above, multiple studies in typically developing populations have demonstrated an overall reduction in structural and functional connectivity between PFC and amygdala from childhood to adulthood (
114,
115). This normative reduction in connectivity in this circuit likely represents a potential neurobiological basis for the improvements in emotion regulation observed over the course of development. However, during the adolescence time frame, there have been divergent reports in human neuroimaging studies of possible transient increases in amygdala-PFC functional connectivity (
90,
116). These studies highlight the need for additional research assessing the transitions that occur during adolescence, when dynamic reorganization of multiple neural circuitry is taking place (
75,
114).
One significant question is whether the neural circuit alterations observed in patients preceded the emergence of the disorder or rather reflect the chronicity or other sequelae of the disorder, such as effects of medications. In this issue of the
Journal, the article by Tromp et al. (
102) addresses this key question by performing detailed DTI studies in unmedicated preadolescent children (ages 8–12) with anxiety disorders and in age-matched control subjects. Based on tract-based DTI analyses, the authors found selectively decreased fractional anisotropy in the uncinate fasciculus in children with anxiety disorders. Interestingly, these neuroanatomical alterations were observed only in boys with anxiety disorders. While previous studies in adults, adolescents, and children with anxiety disorders have reported alterations in the uncinate fasciculus, no sex differences have been reported previously. This study is significant, as it is one of the first to suggest that children with anxiety disorders have an a priori alteration in PFC structural connectivity that cannot be explained by treatment with medications. Moreover, the finding that it is male specific suggests that an increased focus on assessing sex differences is crucial to comprehensively investigating the neural correlates underlying anxiety disorders.
In addition to anxiety disorders, altered cortico-limbic connectivity has been seen in other disorders, such as bipolar disorder (
117) and schizophrenia (
118). As with anxiety disorders, the developmental onset of these neural circuitry changes has yet to be established. The article by Jalbrzikowski et al. in this issue of the
Journal (
103) addresses the emergence of neural alterations in patients with psychotic spectrum disorders. A notable strength of their rsfMRI study is the large number of age-matched control subjects, for a total of 1,062 participants. These rsfMRI data sets were used to construct normative developmental trajectories of amygdala connectivity across late childhood through young adulthood (ages 10–25). The authors were able first to replicate their previous finding (
115) that in normative subjects, there was an overall decrease in centromedial amygdala connectivity with multiple brain regions, including the ventrolateral PFC, dorsolateral PFC, caudate, and thalamus. These findings are consistent with an overall decrease in connectivity shown more broadly between cortical and subcortical structures across development. Having mapped this “growth chart,” the authors then compared centromedial amygdala connectivity with various brain regions in youths with psychotic spectrum disorders. Interestingly, they found that these youths failed to show a developmental decrease in functional connectivity between centromedial amygdala and striatum, thalamus, ventrolateral PFC, and occipital cortex. In fact, at the earliest age (10 years), connectivity between the centromedial amygdala and these other brain regions already appears to be significantly reduced in youths with psychotic spectrum disorders, and there is subsequently little or no additional reduction in connectivity—and in some patients an actual increase in connectivity occurs across the transition to young adulthood. One interpretation of these findings is that in late childhood, patients with psychotic spectrum disorders have undergone a general accelerated developmental decrease in amygdala connectivity, similar to the precocious development of amygdala-centric circuitry observed in individuals who have undergone early-life stress or deprivation and in preclinical rodent models of early-life stress (
29,
30,
41,
42). Conversely, later in development, by young adulthood, patients with psychotic spectrum disorders may have an altered trajectory characterized by increased amygdala connectivity to the lateral PFC, caudate, and occipital cortex, indicating continued alterations of these circuits during the transition into adulthood.
Conclusions
As highlighted in both these articles in this issue of the
Journal as well as previous human neuroimaging and preclinical studies, the maturation of neural connectivity patterns in typically developing subjects, as well as in patients with psychiatric disorders, is a dynamic, nonlinear process that does not occur uniformly in all brain regions. The Tromp et al. (
102) study suggests that in children with anxiety disorders, a key white matter tract, the uncinate fasciculus, displays decreased microstructural integrity, highlighting the possibility that decreased PFC-limbic connectivity may be a key early neuroanatomical hallmark of these disorders. It is particularly interesting that this effect is apparent in boys and not girls. Conversely, in the Jalbrzikowski et al. study (
103), youths in this same late childhood age range who have psychotic spectrum disorders, compared with healthy youths, display reduced amygdala-PFC connectivity that then does not decrease across later development, suggesting a fundamentally different maturation process that may underlie the affective dysregulation that often precedes and predicts increased psychotic symptoms.
While enormous progress has been made in recent years in parsing out the neurobiological and behavioral patterns characteristic of developmental stages from infancy through adolescence, additional work in this area is necessary. In particular, the field is still limited in its understanding of how additional factors such as sex, genetic differences, early-life adversities, and other environmental factors influence the developmental landscape of learning and memory (
14). Research elucidating typical and atypical brain development patterns will be crucial for identifying early-life risk factors that may underlie the emergence of mental illness and provide the key to treating susceptible individuals. Such an effort will depend in large part on optimizing clinical interventions specifically to treat symptoms as they manifest during childhood and adolescence, rather than relying on existing therapies that have largely been optimized for adults. The articles included in this issue of the
Journal provide promising steps in this direction.