Cognitive models for panic disorder posit that biased information processing plays a crucial role in the etiology and maintenance of panic disorder (
1,
2). Normalization of cognitive biases has been proposed to mediate the effect of cognitive-behavioral therapy (CBT) (
3), which is the first-line treatment for panic disorder (
4). Despite their importance for theoretical models, neural correlates of biased information processing and their responsiveness to CBT have rarely been investigated using cognitive experimental approaches.
Schemata with enhanced panic-related associations are a core assumption of cognitive models in panic disorder (
2,
5,
6), such as facilitated panic-trigger-misinterpretation responses, indicating a biased semantic network. On a behavioral level, patients with panic disorder reported stronger associations between body sensations and catastrophizing interpretations (
7,
8). More importantly, in semantic priming experiments, they demonstrated facilitated automatic lexical processing of catastrophic outcomes preceded by somatic symptom words (“breathless-suffocate”) compared with those preceded by neutral, unrelated words (“lemonade-suffocate”) (
9–
11). This suggests an automatic activation of panic-related associations that may have an impact on the very early stages of information processing in panic disorder.
The neural correlates of enhanced panic-related associations in panic disorder are unknown. Semantic priming studies in healthy subjects have demonstrated that strong semantic associations resulted in the facilitation of semantic access, retrieval, and selection of primed neutral target words, with a corresponding activation suppression in the left temporal cortex and inferior frontal gyrus (IFG) (
12–
14). Moreover, emotional compared with neutral semantic associations activated the anterior and posterior cingulate cortices (ACC and PCC) and the superior frontal gyrus (
15). Since fear associative learning appears to be mediated by the ACC (
16), a brain region showing structural and functional alterations in panic disorder (
17), the fear association between panic-related concepts may also activate the ACC in panic disorder, possibly reflecting enhanced fear appraisal. In addition to semantic and emotional associations, the negative valence of panic-related concepts was observed to trigger enhanced activation in brain systems subserving threat processing (e.g., the amygdala, insula, and ACC) in panic disorder (
18).
Recent research has moved further to investigate the mechanism of action of CBT at the behavioral and neural levels in panic disorder (
19). Based on cognitive models, a major target of CBT is patients’ cognitive biases (
3). Studies have reported a substantial change in panic-related cognition after CBT for panic disorder (
20). Furthermore, cognitive change mediated clinical improvements (
21–
23). On the neurofunctional level, a functional near-infrared spectroscopy study (
24) suggested that the attention bias-related prefrontal hypoactivation in panic disorder could not be modulated by CBT. However, studies using whole-brain imaging techniques (e.g., functional MRI [fMRI]) demonstrated CBT effects on the neural function of panic disorder, especially its deep brain structures (
25). Panic disorder patients showed reduced activation in the left IFG during differential fear conditioning (
26) and in the amygdala during the processing of agoraphobic pictures after CBT (
27). fMRI studies of anxiety disorders (e.g., social anxiety, specific phobia) mainly showed that CBT reduced neural activation in limbic structures during symptom provocation or processing of disorder-related stimuli (e.g., pictures) (
25,
28). Nevertheless, these fMRI studies made only a limited contribution to our understanding of the neurofunctional underpinnings of treatment-induced changes in panic-related cognition.
To investigate the neural mechanisms of a biased semantic network in panic disorder and the impact of CBT in modulating this cognitive bias, we applied a previously validated (
29) panic-specific automatic semantic priming paradigm for use in the fMRI scanner. In this paradigm, panic-symptom words (e.g., “dizziness”) were primed by agoraphobic situations such as potential panic-trigger words (e.g., “elevator”). Parallel versions were administered before and after a manualized exposure-based CBT. We expected enhanced associations between panic-trigger/panic-symptom word pairs in panic disorder patients compared with healthy control subjects at baseline, as reflected by explicit ratings (higher relatedness), and implicit behavioral (faster response) and neural priming effects (activation suppression in brain areas related to semantic priming [e.g., the left IFG and temporal cortex] and higher activation in limbic structures and the ACC for enhanced fear processing) for the processing of panic-trigger/panic-symptom word pairs. We hypothesized that after treatment, a normalization of panic-related associations would be observed in panic disorder patients, leading to a reduction in explicit relatedness ratings, implicit behavioral priming effects, and normalized neural activation compared with baseline. (See Figure S1 in section S5 of the
online supplement for a schematic presentation of the hypotheses.)
Methods
Participants
The study was part of the German national research network PANIC-NET II, a multicenter study encompassing five research centers, and it was approved by the Ethics Committee of the Medical Faculty of the Philipps–University Marburg (project no. 171/09) and at all local sites. In total, 125 patients with a DSM-IV-TR diagnosis of panic disorder (
30) and 152 healthy control subjects underwent a semantic priming paradigm during fMRI scanning (a CONSORT flow diagram is provided in Figure S2 in section S5 of the
online supplement). Inclusion criteria for patients were a current DSM-IV-TR primary diagnosis of panic disorder with or without agoraphobia (evidenced by the Composite International Diagnostic Interview); a score ≥3 on the Clinical Global Impressions Scale (CGI); and age between 18 and 65 years. In three of the five study centers, a total of 63 patients were treated with a manualized exposure-based CBT. Two patients dropped out before and 13 during treatment; five withdrew consent for posttreatment fMRI assessment. The remaining 43 patients participated in the posttreatment (“T2” for control subjects) assessment. Fifty-four healthy control subjects underwent measurements at T2. After quality control (see section S6 of the
online supplement), behavioral data sets from 118 patients (21 with panic disorder and 97 with panic disorder with agoraphobia) and 150 healthy control subjects at the baseline/T1 assessment, and 42 patients (nine with panic disorder and 33 with panic disorder with agoraphobia) and 52 healthy control subjects at the posttreatment/T2 assessment were included for analyses of behavioral effects. For the fMRI analysis, matched patients and healthy control subjects (103 pairs at baseline/T1 and 39 pairs at posttreatment/T2) with quality-controlled fMRI data were included. The sociodemographic and clinical characteristics of the final behavioral and fMRI subsamples are summarized in
Table 1 and in Table S1 in section S6 of the
online supplement. The study was approved by the ethics committees of all participating universities. All participants gave written informed consent before participating in the study.
Treatment
The manualized CBT protocol (see section S3 of the
online supplement), which was adopted from our previous study (
31), consisted of psychoeducation, individualized analysis of the patient’s symptoms and coping behavior, generation of a treatment rationale for exposure, and implementation of exposure exercises. CBT was administered in six and 12 twice-weekly sessions in patients with panic disorder and with panic disorder with agoraphobia, respectively. Exposure sessions included interoceptive exposure and, in cases of comorbid agoraphobia, additional in situ exposure to patients’ individual agoraphobic situations. Because the two patient groups received treatments with comparable components and demonstrated significant symptom reduction after CBT, the groups were collapsed in the present study to maximize the sample sizes. Treatments were carried out by trained psychotherapists. Weekly supervision and videotaping of all sessions were used to maintain the integrity of therapy and identify violations of the protocol.
Clinical Assessments and MR Image Acquisition
Clinical assessments were administered before and after CBT by trained assessors (see section S2 of the online supplement). Our primary outcome measures included the Hamilton Anxiety Scale, the CGI, the Panic and Agoraphobia Scale, and the alone subscale of the Mobility Inventory. All MR images were acquired in 3-T scanners according to manualized procedures and underwent standardized quality control measurements. Details on the scanners, technical parameters for fMRI data acquisition, and measures of behavioral and fMRI data quality control are provided in sections S4 and S6 of the online supplement.
Experimental Paradigm
In the semantic priming paradigm (
29), we used two groups of prime words (30 neutral words [N] [e.g., “window”], 30 agoraphobic situations as potential panic-trigger words [T] [e.g., “elevator”]), and three groups of target words (30 neutral words [N] [e.g., “curtain”], 30 panic-symptom words [S] [e.g., “dizziness”], and 60 pseudowords [P] [e.g., “salkom”]). Six conditions were constructed by matching the primes and targets: N–N (neutral-neutral; related); T–N (trigger-neutral; unrelated); N–S (neutral-symptom; unrelated); T–S (trigger-symptom; panic-related); and two pseudoword conditions, N–P (neutral-pseudo) and T–P (trigger-pseudo). Each condition contained 30 distinct word pairs, or trials (see section S8 of the
online supplement). Each trial started with the attention cue “+” (duration, 500 ms) followed by the prime word (duration, 200 ms). The target word then appeared for 1000 ms, followed by a number sign. Immediately after a target word was presented, a lexical decision task was performed. Participants were instructed to press one of two buttons (words compared with pseudowords) as quickly and accurately as possible in response to the target word, using the left index or middle finger.
We used a rapid event–related fMRI design (see section S8 of the
online supplement) (
32). The stimuli display was controlled using a Presentation script file (version 11.0; Neurobehavioral Systems). For adequate parallel versions for the posttreatment assessment after CBT, we developed two sets of stimuli with comparable word pairs and adopted two counterbalanced trial orders with pseudorandomization, resulting in four counterbalanced versions. After the fMRI session, participants rated the valence (from −3, highly negative, to 3, highly positive) and relatedness (from 1, unrelated, to 7, highly related) of the word pairs where the target was a real word.
Behavioral and fMRI Data Analysis
Reaction time was calculated for correct responses only. To eliminate outliers, individual reaction times were eliminated if they exceeded the mean of their condition by more than two standard deviations. To investigate whether patients with panic disorder, compared with healthy control subjects, perceived higher relatedness, greater negative valence, and made faster lexical decisions to T–S (compared with N–S), relatedness (T–S>N–S), valence (T–S<N–S), and reaction time (T–S<N–S) were calculated for each participant. Generalized estimating equation analyses, using SPSS (IBM, Armonk, N.Y.), were applied to our longitudinal data with dropouts. The above-mentioned variables were predicted by group, time, and their interactions. The stimulus set was entered as a covariate because it was not adequately balanced across group and time (χ2=4.55, two-sided p=0.20). Post hoc t tests, using SPSS, with Bonferroni correction for multiple comparisons, were used to examine group differences (panic disorder compared with healthy control subjects) and CBT effects in panic disorder patients (baseline compared with posttreatment assessment). Cohen’s d effect sizes were calculated. (Additionally, analyses of behavioral effects at baseline/T1 are provided in section S10 of the online supplement.)
MRI data were analyzed using standard routines of Statistical Parametric Mapping (SPM8) implemented in MATLAB 7.7. The first five volumes of each functional run were discarded. Standard slice-timing (middle slice), realignment, and normalizing functions (Montreal Neurological Institute template, 2×2×2 mm) of SPM8 were applied. To account for differences in intrinsic smoothness between scanners, an iterative smoothness equalization procedure was performed using a target smoothness of 10 mm full width at half maximum Gaussian isotropic kernel (
33).
For single-subject analyses, realignment parameters were included as regressors of no interest to account for movement artifacts. A high-pass filter with a cutoff period of 128 seconds was applied. The hemodynamic response triggered by the target words in all six conditions was modeled with a canonical hemodynamic response function. The parameter estimate (β) and t-statistic image of N–S>T–S was calculated for each subject. Next, we performed a random-effects group analysis by entering the parameter estimates of N–S>T–S for each group at both time points (panic disorder and healthy control subjects at baseline/T1 and posttreatment/T2 assessments) into a flexible factorial analysis. The fMRI centers were introduced as covariates to account for scanner differences. Further covariates of no interest included sex, age, education level, history of smoking, stimulus set, trial order, and depression (measured with the Beck Depression Inventory–II). To ensure that the covariates did not significantly bias the results, we compared the analyses with and without covariates (see section S13 of the
online supplement). We employed a Monte Carlo simulation to establish an appropriate voxel contiguity threshold (
34). Assuming a single-voxel type I error of p<0.05, a cluster extent of 122 contiguous resampled voxels was indicated as necessary to correct for multiple voxel comparisons at p<0.05 (see section S9 of the
online supplement).
Following our hypotheses, contrasts of interest focused on panic-priming effect (T–S compared with N–S) and its baseline group differences and group-by-time interaction. Because the processing of T–S (compared with N–S) could lead to response suppression in priming-related brain regions or activation enhancement in fear-related limbic structure, we tested both T–S>N–S and T–S<N–S in panic disorder patients (compared with healthy control subjects) at baseline and the changes by time (baseline compared with posttreatment assessment). As supportive post hoc contrasts, we reported the panic-priming effect in the patient group (i.e., without comparisons with healthy control subjects) separately at both time points (see section S12 of the online supplement). Post hoc t tests of significant clusters using their eigenvariates (extracted using the VOI function of SPM8) were conducted to determine whether panic disorder revealed significant activation modification from baseline to posttreatment assessment in these brain regions.
Correlations between the behavioral and neural changes and clinical improvements were calculated to explore the potential mediators of CBT.
Results
Clinical CBT Effects
After CBT, patients had substantially decreased fear symptoms, with effect sizes ranging from 0.37 to 1.26 (see
Table 1; see also Table S3 in the
online supplement).
CBT Effects on Panic-Related Semantic Priming: Behavioral Data
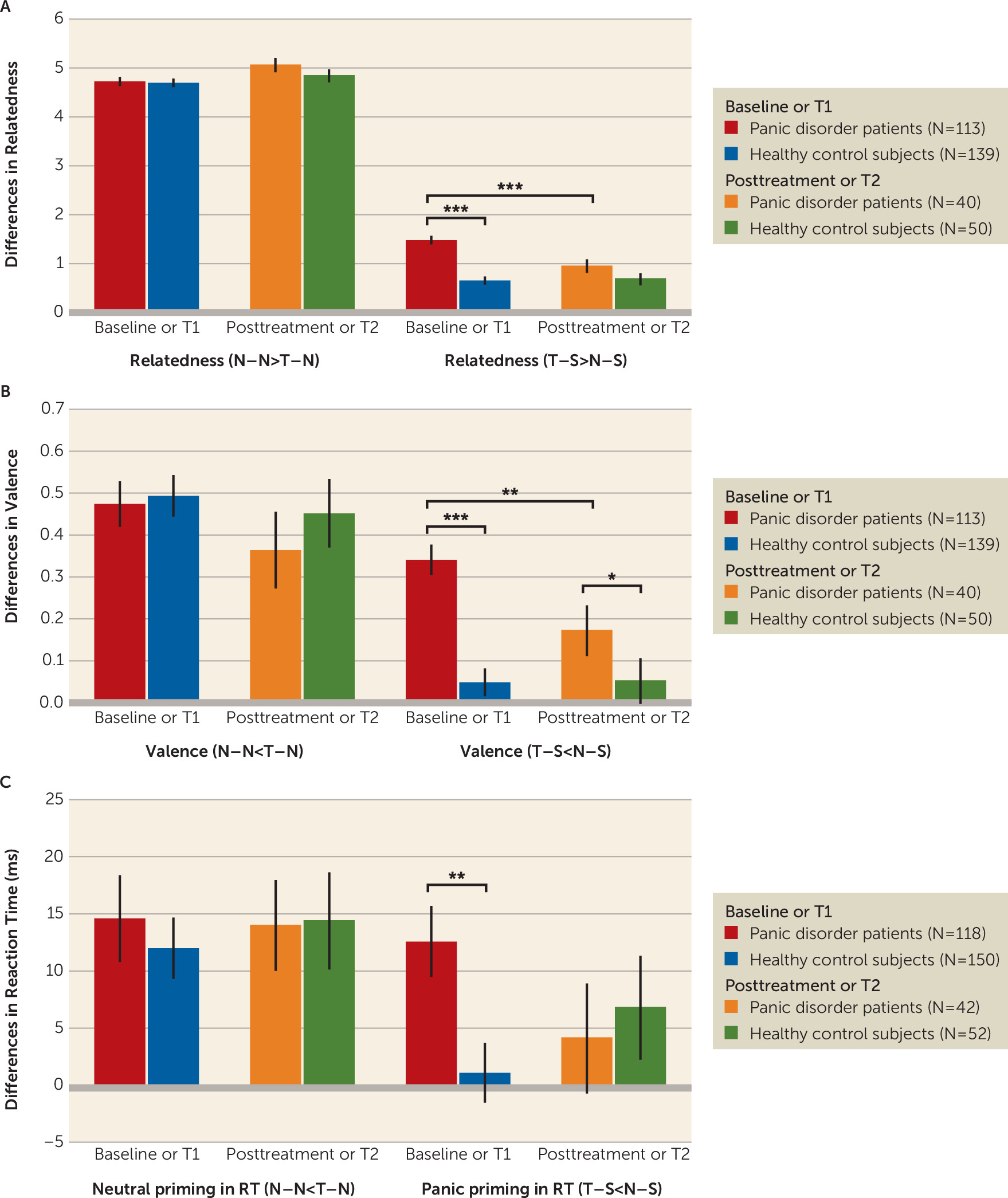
Enhanced relatedness (relatedness [T–S>N–S]).
The group-by-time interaction was significant (χ
2=19.75, N=343, df=1, p<0.001). Post hoc tests indicated higher relatedness for T–S (compared with N–S) in panic disorder compared with healthy control subjects at the baseline/T1 assessment (t=8.53, df=171, p<0.001, d=1.11), but not at the posttreatment/T2 assessment (t=1.81, df=88, p=0.07, d=0.39), and reductions from baseline to posttreatment assessment within patients (t=3.39, df=158, p=0.001, d=0.66) (
Figure 1A).
Higher negative valence (valence [T–S<N–S]).
The group-by-time interaction was significant (χ
2=5.59, N=343, df=1, p=0.018). Post hoc tests indicated higher negative valence for T–S (compared with N–S) in panic disorder compared to healthy control subjects at baseline/T1 (t=6.00, df=162, p<0.001, d=0.78) and posttreatment/T2 assessments (t=2.32, df=88, p=0.02, d=0.50) (
Figure 1B). Furthermore, panic disorder patients reported less negative valence for T–S (compared with N–S) after CBT (t=3.05, df=142, p=0.003, d=0.47).
Higher accuracy (error [T–S<N–S]).
No significant main effect of group (χ2=0.03, N=362, df=1, p=0.86) and group-by-time interaction was detected (χ2=0.01, N=362, df=1, p=0.93).
Facilitated response (reaction time [T–S<N–S]).
The group-by-time interaction fell short of significance (χ
2=2.76, N=362, df=1, p=0.097). Post hoc tests indicated response facilitation (reaction time [T–S<N–S]) for panic disorder compared with healthy control subjects at the baseline assessment (t=2.85, df=266, p=0.005, d=0.35) (
Figure 1C), but not at the posttreatment assessment (t=−0.40, df=92, p=0.69, d=0.08). No significant differences in response facilitation (reaction time [T–S<N–S]) were observed before and after CBT in panic disorder (t=1.36, df=158, p=0.18, d=0.25).
Behavioral results in the fMRI subsample are presented in section S11 of the online supplement. All effects are consistent with the whole sample.
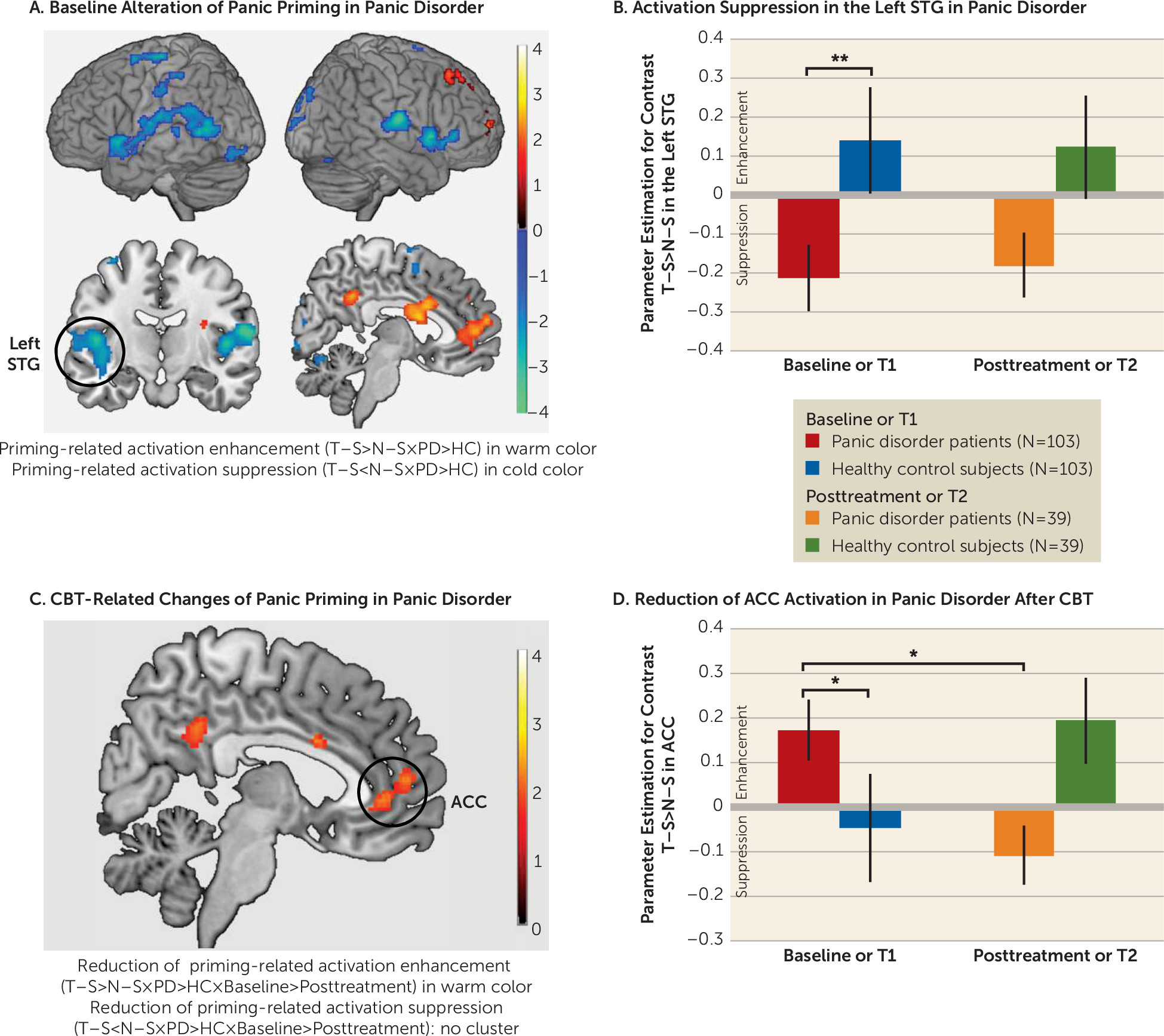
CBT Effects on Panic-Related Semantic Priming: Neurofunctional Data
The fMRI results are presented in
Figure 2 and in section S12 of the
online supplement.
Baseline group differences in panic-priming effect.
At baseline, patients with panic disorder, compared with healthy control subjects, demonstrated panic-priming-related activation enhancement (T–S>N–S) in midline cortices, including the medial prefrontal cortex, ACC (with extension from rostrodorsal to ventral part), middle cingulate cortex (MCC), and PCC/precuneus. Significant panic-priming-related activation suppression (T–S<N–S) was observed in the left and right temporal cortices (e.g., superior and middle temporal gyri), supramarginal gyri, inferior parietal lobule, rolandic operculum, and insula in patients with panic disorder compared with those in healthy control subjects.
Group-by-time interaction in panic-priming effect.
Within the brain regions with baseline group differences, a significant group-by-time interaction was found in the ACC, MCC, and PCC for panic-priming-related activation enhancement (T–S>N–S) (
Figure 2C). No group-by-time interaction was found for panic-priming-related activation suppression (T–S<N–S).
Post hoc tests of CBT effects in panic disorder
After CBT, the panic disorder group exhibited a significant reduction of activation enhancement only in the ACC (t=−2.10, df=140, p=0.04, d=0.39; see
Figure 2D), not in the PCC (t=−0.84, df=140, p=0.40, d=0.15) and MCC (t=−1.83, df=140, p=0.07, d=0.34). To test the insensitivity of panic-priming-related activation suppression of panic disorder to CBT, we extracted the eigenvariates of the significant clusters in the left and right temporal cortices showing baseline group differences. Post hoc tests revealed great similarity of activation suppression at the baseline and posttreatment assessments in the panic disorder group (left: t=0.18, df=140, p=0.86, d=0.03 [see
Figure 2B]; right: t=0.28, df=140, p=0.78, d=0.05).
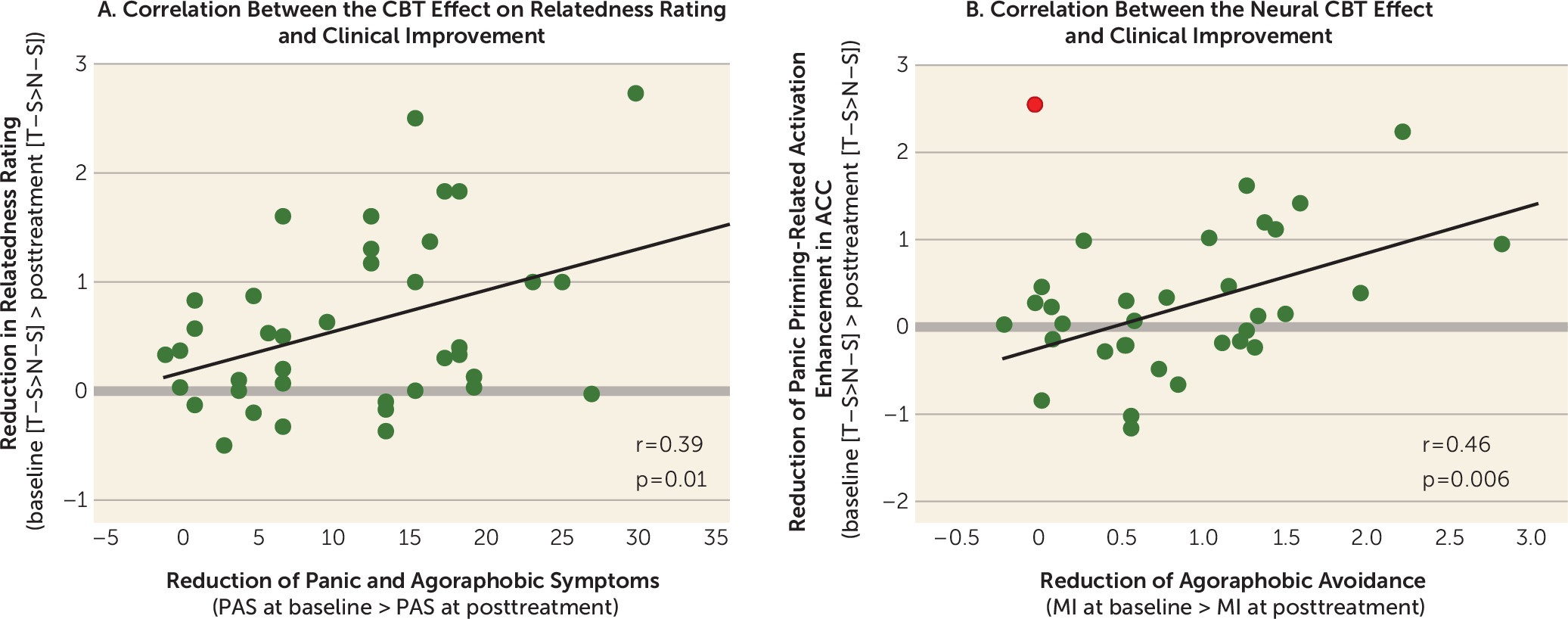
Correlations Between the Behavioral and Neural CBT Effects and Clinical Improvement
An overview of correlations is presented in section S14 of the
online supplement. Reductions in relatedness rating for T–S compared with N–S correlated positively with the reductions in score on the Panic and Agoraphobia Scale (r=0.39, p=0.01) (
Figure 3A) and the alone subscale of the Mobility Inventory (r=0.32, p=0.05). Decreased activation in the ACC correlated positively with reductions in the alone subscale of the Mobility Inventory (r=0.46, p=0.006) (
Figure 3B).
Discussion
In this study, we investigated the effect of CBT on panic-related semantic networks in panic disorder at the behavioral and neural levels. At baseline, altered panic-related semantic networks in panic disorder were supported by explicit ratings, implicit priming-related response facilitations, and neural activations, particularly in the ACC. After treatment, these effects were attenuated in patients, which positively correlated with their clinical improvement. Our results suggest a biased panic-related semantic network in panic disorder, which can be normalized after CBT.
We demonstrated enhanced panic-trigger/panic-symptom associations, the signature of a biased semantic network, in patients with panic disorder using multimodal measurements. Patients with panic disorder perceived stronger relatedness between panic-trigger/panic-symptom words and rated them as having higher negative valence. In the automatic semantic priming paradigm, faster responses to panic-symptom words were observed in patients with panic disorder when they were preceded by panic-trigger words, compared with those preceded by neutral words. This effect was absent in healthy control subjects. According to the spreading activation theory (
35) on semantic priming, response facilitation suggests enhanced panic-related associations, which allows the semantic activation of agoraphobic cues (e.g., “elevator”) in the semantic memory to automatically spread to related panic-symptom words (e.g., “dizziness”), facilitating the retrieval of those concepts. Our results support enhanced interconnections between panic-related concepts proposed by the cognitive models of fear (
2,
5,
6) on behavioral and neural response levels. This priming effect in panic disorder was correlated with activation suppression in the temporal cortex and adjunct insula, regions frequently found to have suppressed activation in previous priming studies with healthy subjects (
13,
14). These findings suggest a reduced effort for lexical access and retrieval of panic-symptom words primed by panic-trigger words in accordance with the behavioral facilitation specific for panic disorder. Furthermore, panic priming in panic disorder was also correlated with activation enhancement in the ACC (both the rostral and ventral parts) and the PCC for panic trigger-symptom word pairs. Both brain regions have been intensively studied, and multiple cognitive and emotional functions have been ascribed to them. Considering our experimental design, with description of agoraphobic triggers, panic symptoms, and their emotional association, we assume that panic trigger-symptom word pairs could have triggered enhanced panic-related episodic memory retrieval (PCC) (
36), fear appraisal (rostrodorsal ACC) (
16), and increased need for implicit emotion regulation (ventral ACC) (
37) in panic disorder. In our previous study with high anxiety-sensitive but healthy subjects, we also found a panic-priming-related activation suppression in the semantic network, including the left IFG, angular gyrus, and fusiform gyrus (
29). Therefore, we propose a trait-like predisposition in the semantic neural network of patients with panic disorder and associated intermediate phenotypes, in which panic-related associations are easily learned or achieved. Of note, high anxiety-sensitive healthy subjects did not show enhanced activation in the cingulate cortex—notably the ACC—for the panic-related condition (T–S>N–S), which could be considered a feature of panic disorder pathophysiology.
Applying a longitudinal design, we provided preliminary evidence that biased semantic information processing in panic disorder normalizes after CBT. Previous behavioral studies validated the mediating role of cognitive changes during CBT in clinical improvement (
22,
23). Our results confirm that CBT has an impact on networks involved in cognitive processing and expand the behavioral evidence to the neural level. Interestingly, the applied treatment consisted of considerable amounts of behavioral exposure, which is thought to induce changes in emotional-associative learning. Hence, we assume that implicit attenuation of panic trigger and panic symptom associations was probably driven by extinction learning processes and, on a more cognitive level, the critical verification of patients’ central concerns during exposure exercises (
38). Surprisingly, the panic-priming-related activation suppression in priming-related brain regions such as the temporal cortices was not attenuated after CBT, although the behavioral priming effect was attenuated. According to the bioinformational theory (
6), we suppose that besides semantic interconnection, an emotional association also exists between the panic-related words. CBT weakened the emotional association and thus reduced the perception of relatedness, the emotional priming-related behavioral facilitation, and the neural activation in the ACC. These processes may have mediated therapeutic effects, as suggested by their correlations with clinical improvements. Combining our previous finding that CBT response in panic disorder was associated with modulation of baseline ACC activation during extinction learning (
39), we propose that the ACC may also be a core target site of CBT for panic disorder and for other disorders from the internalizing spectrum (e.g., depression) (
40). In summary, we demonstrated a normalization of the biased semantic network in panic disorder after CBT.
The strengths of this study include its large sample, a longitudinal multicenter design with manualized CBT, a novel experimental paradigm, and multimodal measurement. The study also has several limitations, however. A higher incidence of tobacco use in the patient group could have biased the neural effects. Attrition led to a selective sample of panic disorder patients. The lack of a waiting-list control group leaves spontaneous symptom fluctuation/regression uncontrolled. Our inferences are only correlative. Further studies are needed to confirm whether the biased semantic network in panic disorder was specifically changed by CBT and mediated the therapeutic changes. To maximize the sample size, we included panic disorder patients without agoraphobia. Post hoc analysis comparing patients with and without agoraphobia showed similar panic-priming effects at both assessment points, indicated by insignificant diagnosis and diagnosis-by-time effects in most of the behavioral and neural effects showing group differences between patients and healthy subjects (see section S16 of the online supplement). Considering the replication crisis in neuroscience and the recent criticism of cluster correction procedures for fMRI studies, the neural effects need replication.
In conclusion, we demonstrated a biased panic-related semantic network in panic disorder and its normalization after CBT on different measurements (for a summary, see section S15 of the online supplement). Convergent results showed enhanced associations between agoraphobic situations (e.g., “elevator”) and the concepts of panic symptoms (e.g., “dizziness”) at baseline and decoupling after CBT in panic disorder. Furthermore, panic-related associations were processed specifically with enhanced activation in the ACC in panic disorder, which were again normalized after CBT. A better understanding of this biased information processing can inform theoretical models on the etiology and maintenance of panic disorder and foster the development of innovative behavioral treatment strategies.
Acknowledgments
The authors thank Nina I. Kleint, Katharina Holtz, Özkan Genc, Johanna Gechter, Kirité Rugani, and Anja Balser for their assistance with fMRI data collection.