Individuals in recovery from alcohol use disorder (AUD) struggle with poor treatment adherence, early dropout, and high risk of relapse (
1,
2). Furthermore, patients with AUD enter outpatient treatment with varying lengths of abstinence, from less than 24 hours up to a few weeks (
3). Little is known about the neurobiological state during early abstinence, a critical time for outpatient treatment initiation. Current research on brain recovery with abstinence from alcohol includes evidence from animal models (
4–
6) and data from a select group of AUD patients who can abstain for months or years at a time (
7–
10). However, over 35% of AUD patients entering outpatient treatment relapse within 30 days, and over 65% relapse within 90 days (
1,
11,
12). Furthermore, the effects of each day of alcohol abstinence on functional brain recovery and the impact of such recovery on lapses to heavy drinking during early treatment are not known.
Previous research indicates that chronic alcohol intake is associated with neuroadaptations in functional brain responses in stress and reward circuits (for reviews, see references
13,
14). These neuroadaptations interfere with adaptive emotion processing, stress regulation, and cognition (
15) and are associated with high alcohol craving, thereby increasing susceptibility to continued heavy drinking and high relapse risk after treatment (
11). Specifically, we have shown (
16) that in AUD patients who have been abstinent for 4 weeks, disrupted activity of the ventromedial prefrontal cortex (vmPFC), encompassing the orbitofrontal cortex (OFC) and the rostral anterior cingulate cortex (rACC), and ventral striatal responses to stress, alcohol cue, and neutral-relaxed conditions was associated with higher stress- and alcohol-cue-induced craving and increased relapse risk after inpatient treatment. While the vmPFC and ventral striatal regions make up the salience network and are identified as a key circuit in reward prediction (
17,
18) in addiction models (
19), the vmPFC and ventral striatum have been shown to be responsive to stress and negative mood cues (
20) and predictive of resilient and adaptive coping under stress (
21). The effect of days of alcohol abstinence on the integrity of brain functional responses of this key prefrontal-striatal circuit known to predict alcohol relapse has not been systematically investigated thus far.
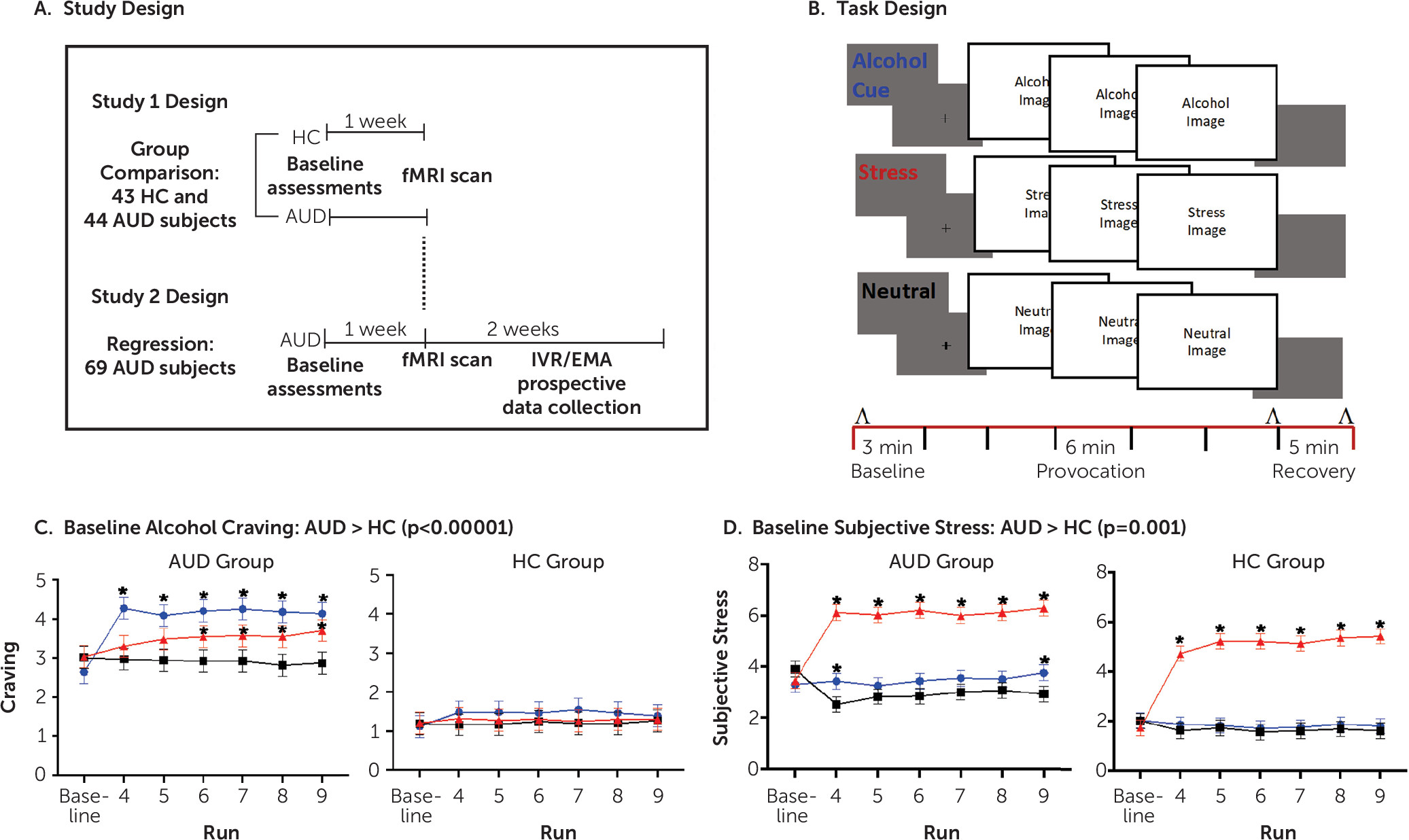
To address this gap, we conducted two related studies utilizing a unique multimethod functional MRI (fMRI) experimental approach combined with a prospective clinical outcome design. A novel sustained stress and reward provocation task employed a series of standardized and matched visual cues for alcohol, stress, or neutral stimuli over a brief period in a block design (
22). Study 1 assessed the brain functional responses to stress images and to alcohol cues relative to an active neutral-control cue block in 44 treatment-entering AUD patients and 43 demographically matched control subjects to specifically examine possible functional disruption of the prefrontal-striatal circuit. Study 2 utilized an expanded group of the treatment-entering AUD patients (N=69) to assess whether the fMRI responses to stress images and to alcohol cues in AUD patients are influenced by number of days of abstinence at treatment entry, and whether such abstinence-related neurobiological functioning is predictive of subsequent heavy drinking during the 2-week period after treatment initiation. Based on previous research (
16,
23), we hypothesized in study 1 that the treatment-entering AUD patients would show altered vmPFC and ventral striatal response to the alcohol, stress, and neutral conditions relative to demographically matched, social-drinking healthy control subjects. In study 2, we hypothesized that the extent of functional brain alterations in the vmPFC and ventral striatal regions to stress images and to alcohol cues would be significantly associated with days of alcohol abstinence and that brain changes related to days of abstinence would prospectively predict heavy drinking days during the first 2 weeks of treatment engagement.
Discussion
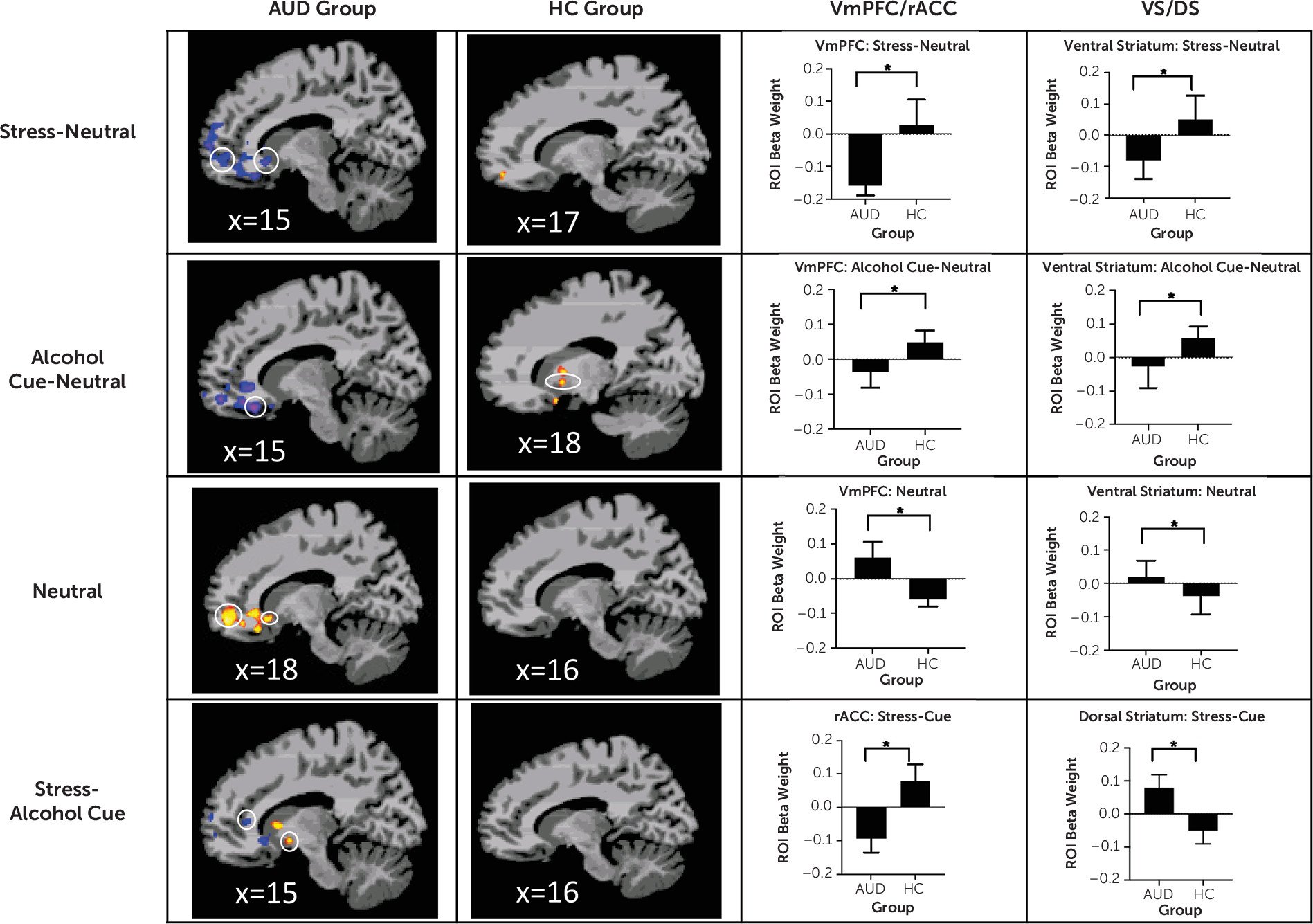
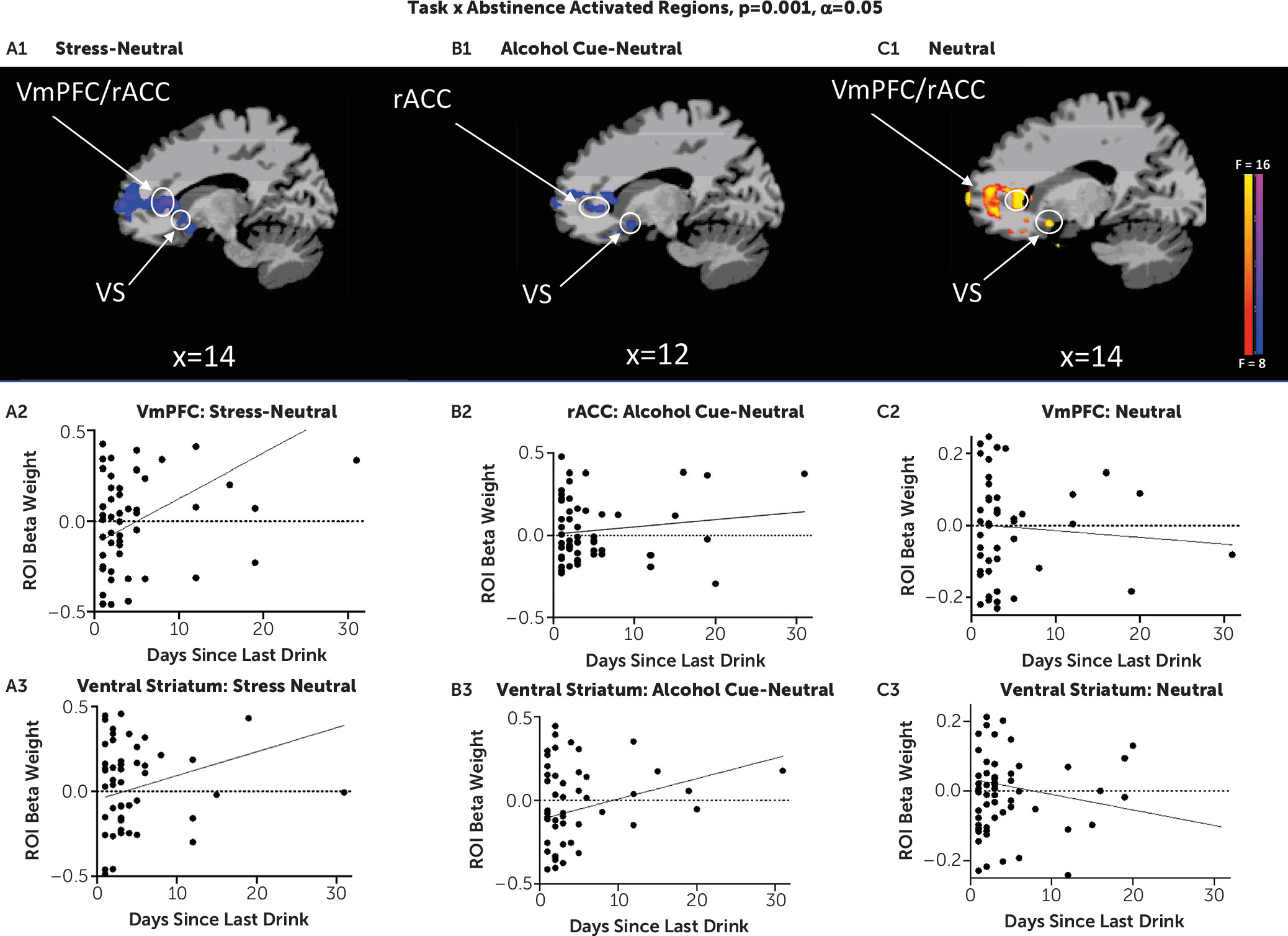
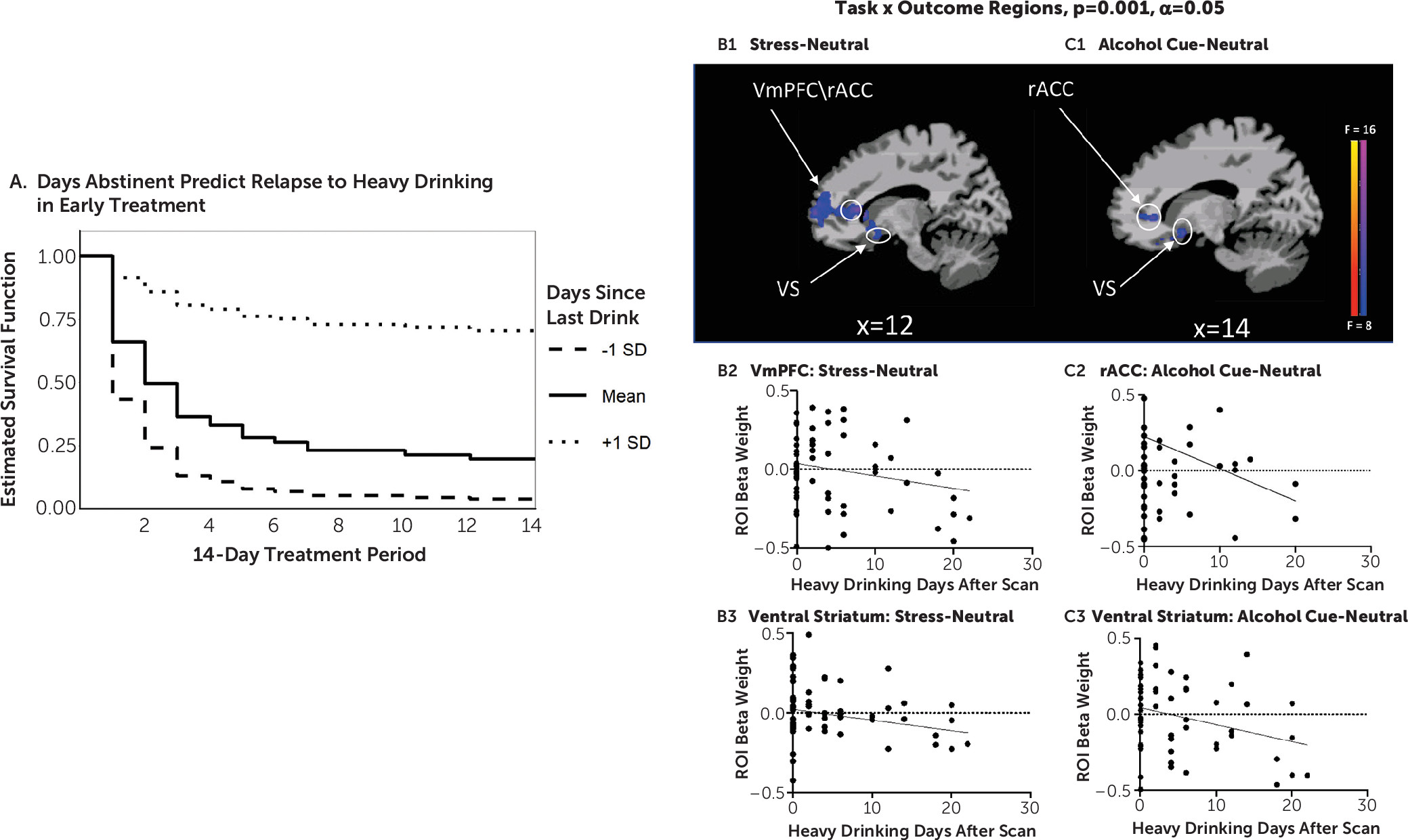
In two related studies, using a novel brief sustained-exposure stress, alcohol cue, and neutral control visual stimuli paradigm, we provide previously unreported evidence of 1) disrupted prefrontal-striatal functional responses to stress and alcohol cue provocation and to the neutral control condition in participants with AUD entering treatment relative to healthy social drinkers, and 2) a significant influence of number of days of alcohol abstinence on extent of disrupted prefrontal-striatal function in response to stress, alcohol cue, and neutral provocation, which in turn had a significant prospective effect on drinking outcomes in early treatment. In study 1, AUD patients, on treatment entry, showed highly significant dysfunctional blunted vmPFC and ventral striatal responses to stress images and to alcohol cues relative to neutral images as compared with demographically matched control subjects but vmPFC/rACC and ventral striatal hyperactivation to the neutral condition. These results supported our hypothesis of significant prefrontal-striatal functional pathology during early abstinence in treatment-entering AUD patients. In study 2, we found that number of days of alcohol abstinence at treatment initiation significantly affected the altered vmPFC/rACC and ventral striatal functional response, such that lower number of days of abstinence prior to the scan was associated with greater disruption of the vmPFC/rACC and ventral striatal activity in response to the stress, alcohol, and neutral conditions. Additionally, after controlling for the effects of abstinence days on brain responses to cues, we found that greater dysfunctional prefrontal and striatal responses to stress and to alcohol cues prospectively predicted more heavy drinking days in the first 2 weeks of outpatient treatment for AUD.
It is notable that dysfunctional prefrontal-ventral striatal functional responses were found in the AUD group relative to the control group in study 1, and that such dysfunction was also related to important clinical indicators, such as number of days of abstinence at treatment entry and drinking outcome during early treatment engagement. We used a whole brain analytic approach to specifically assess the hypothesized prefrontal-ventral striatal circuit across the two studies. The similarity of brain findings across the two related studies speaks to the strength and robustness of the results. Furthermore, while craving, subjective stress, and cortisol response were each correlated with heavy drinking day outcome, only number of abstinence days and abstinence-related dysfunctional brain responses predicted drinking outcome in early treatment. These findings suggest that extent of brain functional pathology along with days of abstinence may be important markers of early treatment outcome and recovery.
Because relapse to drinking and treatment dropout during early outpatient treatment are common, identifying predictors of heavy drinking and relapse during early recovery is critical. Stress and alcohol cues in the environment are two main reasons patients cite for relapse (
29,
30). These results are consistent with our current understanding of the underlying neurobiology of AUD, in which chronic alcohol-related neuroadaptations in prefrontal-ventral striatal circuits may hamper a person’s ability to recruit prefrontal executive control functions in response to stress cues, while also showing hypoactivation in the ventral striatal, appetitive brain regions to respond appropriately to alcohol rewarding stimuli, and this combined dysfunction may increase risk of having heavy drinking days and risk of relapse (
31). This is also consistent with growing evidence that AUD patients are unable to appropriately regulate responses to stress and alcohol cues in the environment during recovery initiation (
29). Additionally, a number of previous studies have reported down-regulation of dopamine and blunted ventral striatal responses to alcohol and to alcohol cues in AUD patients and in binge drinkers or heavy drinkers (
32–
34). Furthermore, we have previously shown ventral striatal hyperactivation to neutral cues in AUD patients (
16), suggesting a heightened tonic salience of alcohol, and probability of approach behavior in the presence of alcohol cues (
35,
36), consistent with previous work in AUD patients in fMRI and PET studies (
13,
37). Dysfunctional ventral striatal responses predicting future alcohol use outcomes are also consistent with previous data on prediction of drinking outcomes in the laboratory (
38) as well as in real-world settings (
39).
Findings from study 2 corroborate those of study 1 in that disruption of prefrontal-striatal functioning in AUD patients was significantly associated with fewer days of abstinence—that is, greater prefrontal hypoactivation during stress, but greater hyperactivity during neutral states, and greater ventral striatal hypoactivity to alcohol cues were associated with lower number of days of abstinence prior to treatment entry. These findings support animal studies that suggest that the brain structural and functional state is highly dynamic during early recovery (
5,
40). Indeed, it appears that with each day of abstinence, there is improvement in functioning of prefrontal-striatal circuits known to be critical for executive control, resilient coping, and regulation of craving and reward responses (
22,
41–
43). Although these findings and others (
44) suggest that longer abstinence periods may be required to reverse disrupted vmPFC and ventral striatal functioning, a critical clinical issue is that such chronic alcohol-related disruptions have a significant impact on heavy drinking lapses and thus may jeopardize initiation of early recovery by increasing risk of relapse.
Our findings suggest the need for development of targeted therapeutics to reverse such prefrontal-striatal dysfunction early in treatment—that is, treatments that aim to reverse vmPFC/rACC and ventral striatal functional disruptions in order to support successful initiation of recovery. Noradrenergic compounds, such as prazosin and guanfacine, which are known to rescue prefrontal stress pathways (
45,
46), have the potential to restore vmPFC/rACC functioning and executive control as well as to reduce stress and drug craving in patients with substance use disorders (
47,
48). Additionally, intensive outpatient or partial hospitalization programs that provide higher levels of support and counseling to maintain alcohol abstinence may also further improve vmPFC functioning to counter high-risk stress situations and alcohol-related contexts during early recovery.
This study has several strengths, including careful assessment of the individual variation in length of abstinence and daily mobile health assessment of drinking behavior during initial treatment engagement to obtain daily data on drinking outcome without introducing bias from poor recall (
49). Another important strength was the validation of a novel standardized visual stress and alcohol cue provocation paradigm in a patient sample, which can be easily implemented and replicated by other laboratories and in different patient samples.
These novel findings must be considered in light of the study limitations. First, our sample size limited the ability to systematically assess sex differences in the sample. Second, it is important to replicate this research in nonsmoking patients with AUD, as different patterns of co-occurring drug use are common among individuals with AUD and may limit the generalizability of our findings. Third, because AUD is associated with higher levels of chronic stress and traumatic experiences, samples with varying levels of trauma and stress exposure may elucidate differential functional dysfunction in AUD patients with and without trauma. Finally, given the challenges of achieving and maintaining alcohol abstinence, future studies also need to consider the effects of significant reductions in alcohol use compared with complete abstinence on early brain recovery.
Despite these limitations, our findings in this study are the first to show a direct relationship between lower number of alcohol abstinence days and greater disruption in prefrontal-striatal function in response to stress, alcohol cue, and neutral conditions, which in turn are predictive of subsequent heavy drinking during early treatment. These findings support the critical need for developing targeted medications and interventions that will facilitate rescue of disrupted prefrontal-striatal functioning to initiate early brain recovery from AUD.