The search for a structural basis of psychopathology in the brain has historically been dominated by case-control studies in which comparisons are made between groups of individuals with or without a specific psychiatric diagnosis (
1). While such studies have reported a multitude of structural brain differences between case and control subjects, they have generally failed to identify specific differences in brain structure that are unique to one diagnosis. On the contrary, the results from these studies generally reveal structural features of the brain that are highly conserved across many disorders (e.g., reference
2). For example, a meta-analysis of neuroimaging data from 15,892 individuals across six categorical disorders identified transdiagnostic structural deficits within attentional and cognitive control networks (
3). A second meta-analysis of data from 14,027 patients identified transdiagnostic structural deficits in multiple cortical regions across eight categorical disorders (
4). Thus, it appears that categorical disorders may not systematically differ in their patterns of associated structural brain alterations.
A similar convergence has been documented in research on psychiatric nosology, where accumulating evidence demonstrates that sets of disorders or symptoms predictably co-occur and can be captured within broader families of disorders (
5,
6). For example, depression and anxiety emerge in the same individuals and comprise the internalizing family; and antisocial behavior and drug abuse emerge in the same individuals and comprise the externalizing family. Recent work has shown that disorganized thoughts, delusional beliefs, hallucinations, obsessions, and compulsions emerge in the same individuals over time and comprise the thought disorder family (
7). In addition, a single general psychopathology factor, often called the
p factor (
7), has been identified that robustly captures the shared variance across these three broader families of common mental disorders (
8–
10). It is thus possible that the aforementioned convergence of structural brain alterations across categorical disorders reflects the pervasive co-occurrence of psychiatric disorders in individuals, which can be indexed by the
p factor. Indeed, a recent study reported global gray matter volume reductions not only across categorical disorders but also with higher general psychopathology (
11).
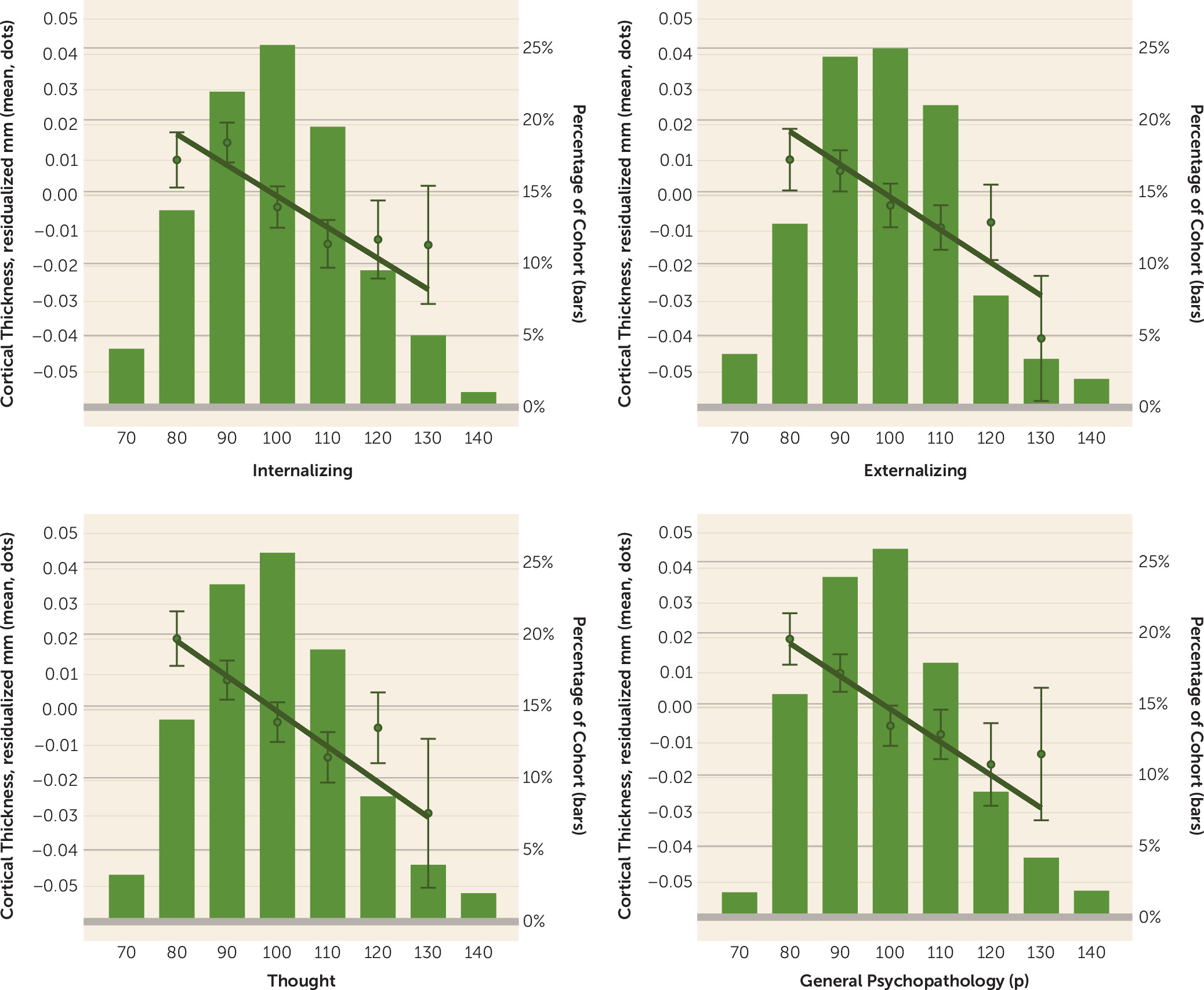
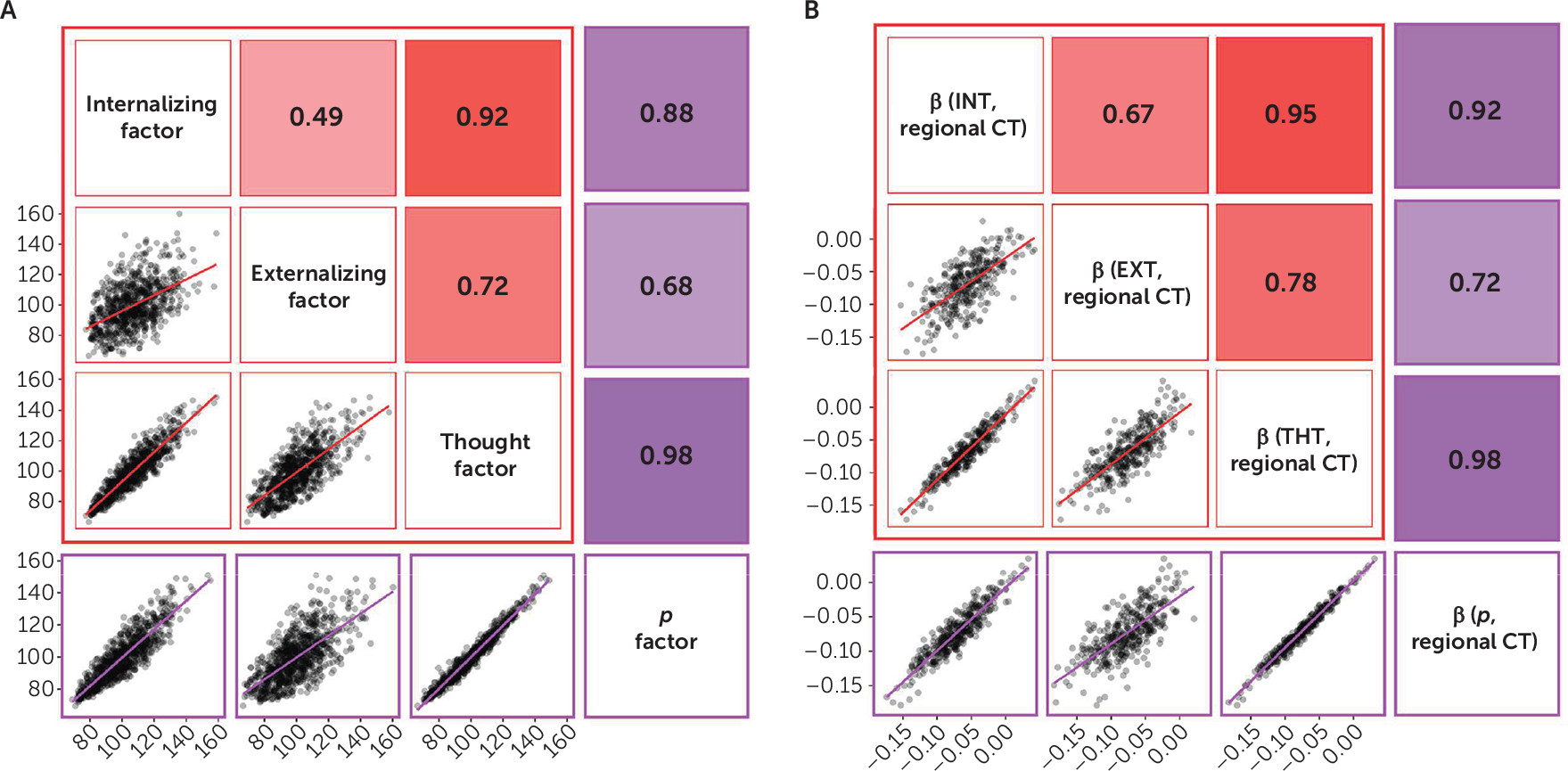
In the present study, we hypothesized that if general psychopathology drives the convergence of structural alterations common across disorders, then 1) there should be few associations unique to any one of the three diagnostic families of disorders, and 2) associations with the
p factor should overlap with those for the three diagnostic families. We tested our hypotheses through variability in two structural features of the neocortex—cortical thickness and surface area—derived from high-resolution structural MRI data collected from members of the Dunedin Study, which has followed a population-representative birth cohort for five decades. Our focus on the neocortex reflects both the preponderance of previous transdiagnostic neuroimaging findings in cortical regions (
3,
4) and the preferential role of cortical circuits in supporting higher-order integrative and executive processes, in which dysfunctions are hypothesized to be a core feature of general psychopathology (
10).
Discussion
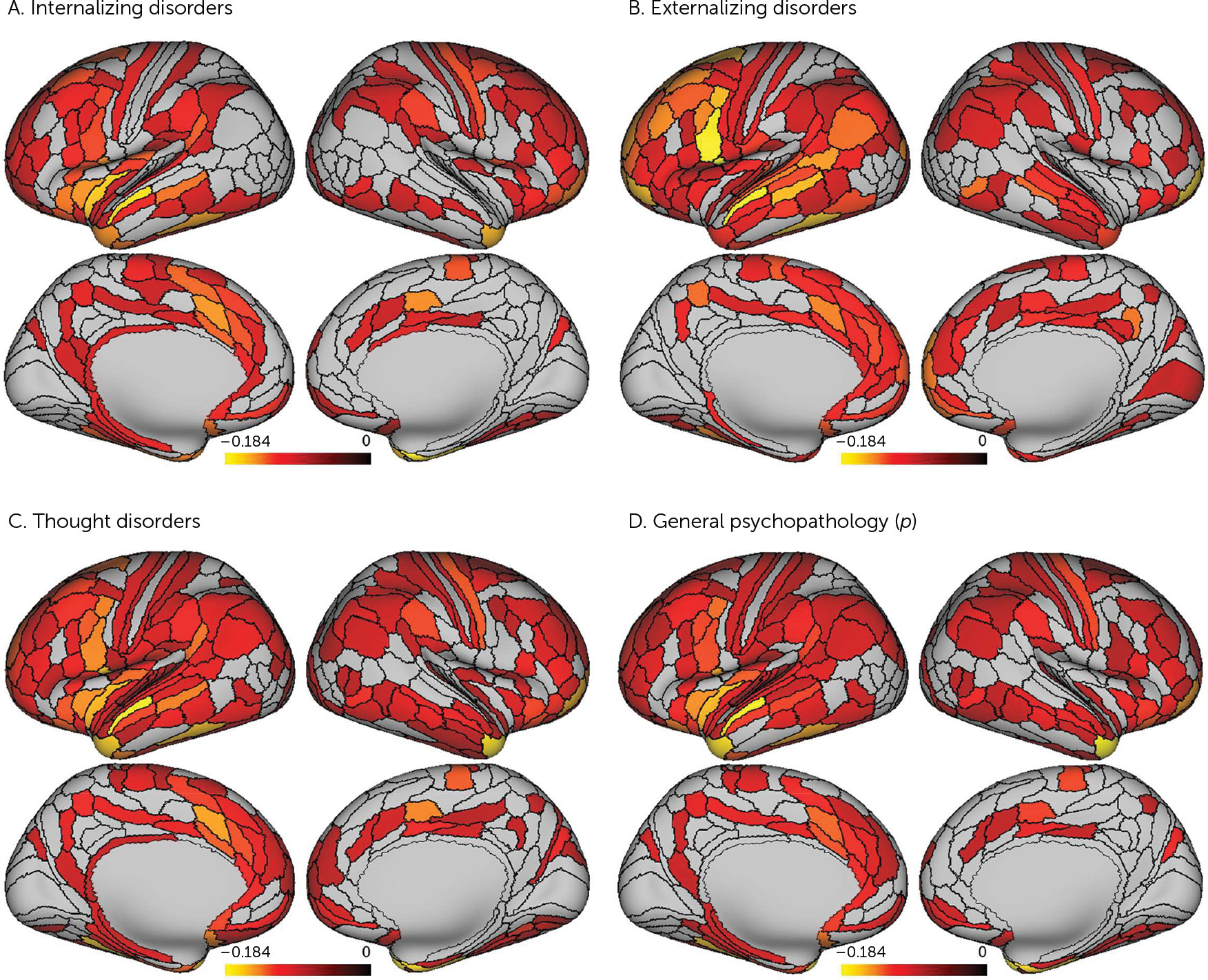
Our analyses suggest that there is little specificity in the brain structural correlates of mental disorders. Rather, a nonspecific and pervasive pattern of thinner neocortex appears to be a transdiagnostic feature of general psychopathology as indexed by the
p factor. Consistent with our hypotheses that general psychopathology (
p) drives the convergence of structural alterations common across disorders, we found 1) very few associations unique to the internalizing, externalizing, or thought disorder diagnostic families and 2) that associations with the
p factor highly overlapped with those for the three diagnostic families. The pervasive and transdiagnostic nature of these associations is consistent with studies revealing that most structural brain differences are not unique to categorical mental disorders but rather are shared across disorders (
3,
4,
11).
The present findings help to refine the interpretation of recent studies that have identified widely distributed reductions in neocortical gray matter volumes associated with higher general psychopathology (
10,
11,
22). Specifically, our findings suggest that these previously reported associations may be driven by reduced neocortical thickness and not surface area, which together comprise gray matter volume. Unlike the broad mapping of reduced global and parcel-wise cortical thickness onto psychopathology, there were no significant associations with parcel-wise neocortical surface area. While there was a significant association with reduced global surface area, this was restricted to higher scores only on the externalizing factor. This is consistent with previous research showing that externalizing disorders tend to load least strongly on the
p factor (
7,
23). As we did not hypothesize this effect, and it could arise from chance, we refrain from extensive discussion. It raises the question of whether this specific association reflects early-life vulnerability to externalizing disorders as a result of differences in the developing brain (e.g., due to genetics or maltreatment) or a result of harm done to the brain from an externalizing lifestyle (e.g., due to drug use, sexually transmitted diseases, or violent injuries, including concussions) (
24,
25). Regardless, the effect sizes associated with either global or parcel-wise surface area, irrespective of statistical significance, were an order of magnitude smaller than those observed for associations with global and parcel-wise cortical thickness.
Thus, the general pattern of associations suggests that reduced cortical thickness and not surface area is a transdiagnostic feature of mental disorders reflected in general psychopathology. Consistent with this pattern, a recent cross-disorder genome-wide association study identified four common variants predicted to affect the function of radial glia and interneurons critical for the development of cortical layers, which are reflected in cortical thickness (
26). That said, the cytological and histological basis of cortical thickness remains unclear. MRI-derived estimates of cortical thickness do accurately reflect the width of the cortical mantle as determined on postmortem examination (
27). Differences in cortical thickness, however, are unlikely to reflect numbers of neurons or loss of neurons over time (
28). Rather, the thickness of the cortex likely reflects a combination of neuron size (i.e., degree of shrinkage) and dendritic arborization (i.e., degree of branching, spine density).
A thinner neocortex has been associated with a host of negative outcomes across the lifespan. For example, a thinner neocortex has been associated with lower intelligence in midlife (
29) and is a feature of older brain age relative to chronological age, which is accompanied by greater cognitive impairment (
30). A thinner cortex has also been observed in categorical mental disorders spanning the internalizing, externalizing, and thought disorder diagnostic families (e.g.,
31–
33). Meanwhile, accelerated thinning has been associated with worsening daily functioning and other symptoms in Alzheimer’s disease (
34,
35). Our secondary analyses of network enrichment are relevant here as they highlight preferential associations between higher
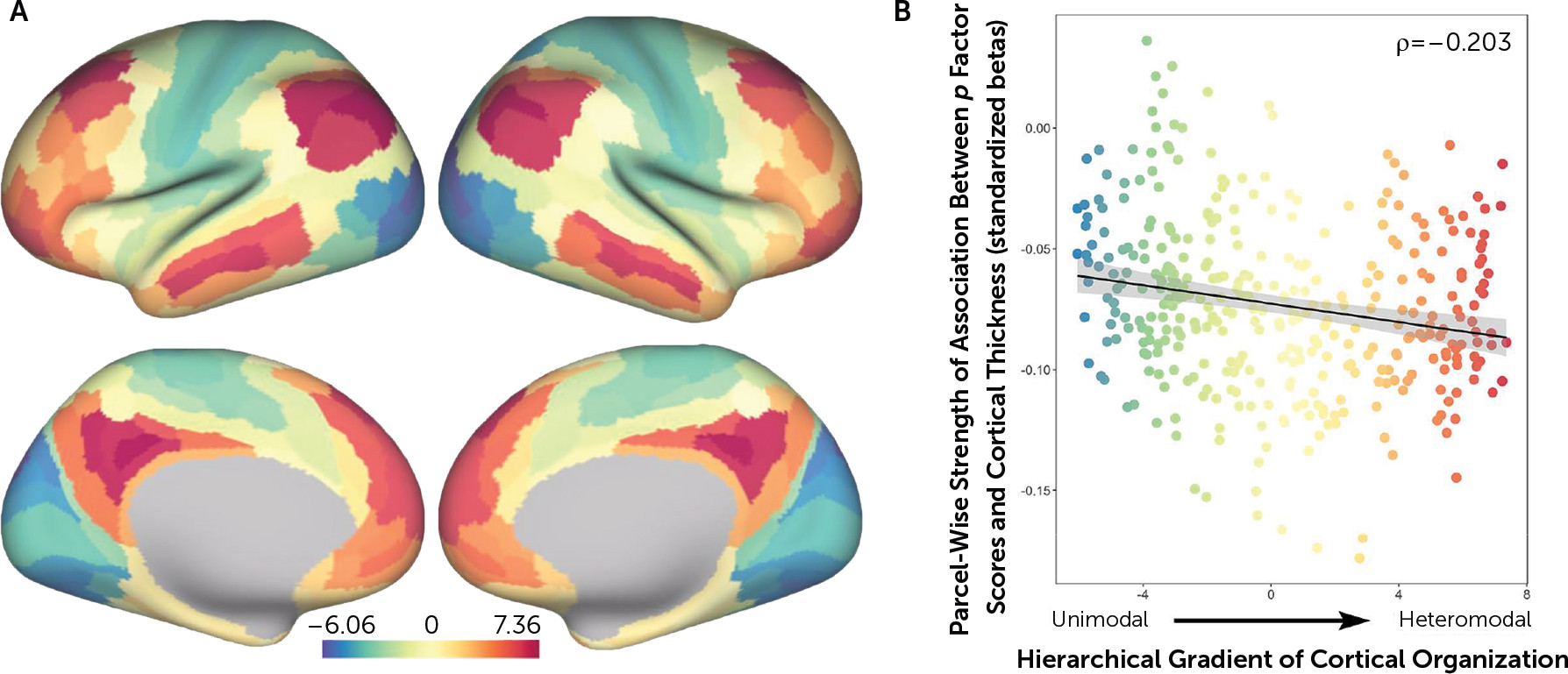
p factor scores and reduced cortical thickness of parcels falling within heteromodal association cortices, including the frontoparietal and default mode networks, supporting higher cognitive processes and executive functions (
36). Thus, while a pervasive pattern of reduced neocortical thickness may be a transdiagnostic feature of general psychopathology, it is possible that specific reductions in heteromodal association cortices (i.e., frontoparietal and default mode networks) may feature in the disordered form and content of thought hypothesized to represent the core of the
p factor (
10,
37). This is consistent with previous transdiagnostic research indicating alterations within frontoparietal and default mode networks supporting executive control and self-referential processes across diagnostic categories (
2,
4,
36,
37). Further evidence comes from parallel neuroimaging studies implicating structural alterations within a cerebello-thalamo-cortical circuit supporting executive functions in the expression of the
p factor (
38–
40).
One limitation of this study is the availability of only a single, cross-sectional assessment of brain structure in midlife. Unfortunately, we have only recently been able to collect neuroimaging data in the Dunedin Study and we are unable to examine temporal order in the data. It remains to be determined whether a higher burden of psychopathology contributes to a pervasively thinner neocortex or if a pervasively thinner cortex contributes to a higher burden of psychopathology. Longitudinal collection of neuroimaging data beginning in early childhood and continuing throughout life is needed to address this question. Comparisons with other cross-sectional studies, as well as longitudinal data, are necessary to determine the extent to which the patterns observed here, at age 45, generalize across development or the extent to which associations between psychopathology and brain structure change across the lifespan. Second, while the Dunedin Study cohort is a population-representative birth cohort free of the selection biases often present in neuroimaging research, it is a predominantly white cohort born in the 1970s in one part of the world; therefore, results will require replication in other samples from other ethnic groups and countries.