Cigarette smoking (
1) and heavy alcohol use (
2) are leading causes of morbidity and mortality in the United States and worldwide. Alcohol use and smoking have high rates of co-use, such that approximately 20%−25% of current smokers are considered heavy drinkers (
3,
4). Heavy-drinking smokers experience more negative health consequences, such as greater risk for various cancers (
5), as well as a lower likelihood of successful smoking cessation (
4,
6–
8). Notably, both smoking (
9) and heavy drinking (
10,
11) have been associated ith worse outcomes for COVID-19, highlighting the importance of treating both conditions. It is estimated that smokers are up to four times more likely to experience a smoking lapse during drinking episodes in the course of smoking cessation treatment (
7). While heavy-drinking smokers constitute a sizable subgroup with a higher risk of negative consequences and failed quit attempts, there are no available pharmacological treatments tailored to heavy-drinking smokers (
12,
13).
Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, is the most effective pharmacotherapy for smoking cessation (
14), with an estimated 1 in 8 patients being abstinent from smoking at 1-year follow-up (
15). Studies have shown that in addition to its smoking cessation benefits, varenicline may also reduce alcohol consumption (
16–
18). Naltrexone, an opioid receptor antagonist, is approved by the U.S. Food and Drug Administration (FDA) for the treatment of alcohol use disorder (AUD) (
19,
20), and it has been shown to increase smoking cessation rates, particularly among heavy-drinking smokers (
21,
22). While varenicline and naltrexone have FDA approval for nicotine and alcohol use disorders, respectively, there is evidence that their clinical benefit may extend across the two disorders.
Toward improving treatments for heavy-drinking smokers, our laboratory previously conducted an experimental medicine study (
23) in which we compared the effects of varenicline only, naltrexone only, the combination of varenicline and naltrexone, and matched placebo. The combination of varenicline and naltrexone was superior to placebo, and at times superior to monotherapy, in attenuating cigarette craving, cigarette and alcohol subjective “high,” and reducing ad libitum consumption of cigarettes and alcohol. The combination of varenicline and naltrexone was associated with the greatest reduction in neural activation of the left and right anterior cingulate cortex during exposure to cigarette cues (
24). These findings suggested that a clinical trial of the combination of varenicline and naltrexone for heavy-drinking smokers was warranted. Combination pharmacotherapy for addiction is an understudied area, with potential to reach additive or even synergistic effects on craving and substance use (
25). Further, the concurrent treatment of smoking and heavy alcohol use holds great promise for patients and health care systems alike (
26,
27).
We conducted a double-blind parallel-group placebo-controlled randomized trial of treatment with varenicline plus placebo compared with varenicline plus naltrexone in heavy-drinking smokers seeking treatment for smoking cessation and drinking reduction. The primary aim in this superiority trial was to test the hypothesis that the combination of varenicline and naltrexone would be superior to varenicline alone with regard to 1) bioverified smoking cessation rates at 26 weeks, and 2) reductions in drinks per drinking day during the 12-week treatment phase and at 26 weeks.
Methods
Trial Design
This was a 26-week randomized clinical trial of varenicline alone compared with varenicline plus naltrexone for smoking cessation and drinking reduction in a community sample of heavy-drinking smokers. After screening and randomization to one of the two study medication arms, participants set a quit date for smoking during a 30- to 45-minute counseling visit with a master’s-level clinician. Consistent with guidelines for the clinical use of varenicline, patients were instructed to quit smoking after reaching the target dosage of the study medication. Study visits occurred at 4, 8, and 12 weeks after quit date, and posttreatment follow-up visits occurred at weeks 16 and 26. This trial was approved by the institutional review board of the University of California, Los Angeles (UCLA) and was monitored by a data and safety monitoring board. All study participants provided written informed consent after discussing the study medications with a licensed physician. Participants were enrolled, randomized, and followed in the study between July 2015 and December 2019. Data analysis was conducted from January to April 2020.
Setting and Participants
The trial was conducted at an outpatient research facility at UCLA. Participants were recruited through print, radio, social media, and mass transit advertisements. After initial telephone or online screening for eligibility, participants were invited for an in-person intake appointment. Eligible participants were men and women ages 21–65 seeking treatment for smoking cessation and expressing a desire to reduce or quit drinking. Participants were required to be smokers who smoked five or more cigarettes per day and to have a breath CO reading ≥4 ppm to verify smoker status (
28). While six participants did not meet the baseline CO level requirement, their smoking status was confirmed by a cotinine test indicating that they were regular smokers. Cotinine was assessed using a urine cotinine test (NicAlert), a rapid test utilizing semiquantitative immunoassay technology; a score ≥3 (100–200 ng/mL) indicates recent tobacco exposure. Participants were also required to meet the National Institute on Alcohol Abuse and Alcoholism definition of heavy drinking (
29), which for men is >14 drinks per week or ≥5 drinks per occasion at least once per month over the past 12 months, and for women, >7 drinks per week or ≥4 drinks per occasion at least once per month over the past 12 months. Exclusion criteria were a lifetime history of a psychotic or bipolar disorder and clinically significant alcohol withdrawal, indicated by a score ≥10 on the Clinical Institute Withdrawal Assessment for Alcohol–Revised (CIWA-Ar) (
30). Participants with current substance use disorder or major depressive disorder with suicidal ideation were excluded from the trial. Women of childbearing age had to be practicing effective contraception and could not be pregnant or nursing. A physical examination (including vital signs), ECG, and laboratory tests were conducted to ensure medical eligibility; participants whose examination and testing revealed any abnormalities were excluded and were referred to their personal physicians for evaluation.
Interventions
A stratified randomization list was developed by the study statistician, based on sex, drinks per drinking day, and cigarettes per day. Participants were randomly assigned to one of two interventions: 2 mg/day of varenicline plus 50 mg/day of naltrexone, or 2 mg/day of varenicline plus matching placebo pills. Pfizer, Inc., supplied the varenicline for the study, and the UCLA research pharmacy prepared all study medications in blister packs, which were dispensed once monthly for the 12-week medication treatment phase. Participants, providers, and research staff were kept blind to medication assignment for the duration of the study. Medication titration for varenicline followed the recommended procedure in its FDA indication for smoking cessation, namely, 0.5 mg once daily for 3 days, 0.5 mg twice daily for 4 days, and 1 mg twice daily for the remainder of the 12-week treatment phase. For naltrexone, participants took 25 mg once daily for the first 5 days and 50 mg once daily for the remainder of the 12-week treatment phase. Study medications were tapered off after week 12. Participants were instructed to take both medications at the same time, and individuals reporting any significant side effects were counseled via telephone by the study physician. Medication adherence was monitored through pill counts from blister packs returned at each study visit. Dosage reductions were allowed, and participants who discontinued the medication could continue to attend research sessions.
At randomization, participants received a 30- to 45-minute counseling session tailored to heavy-drinking smokers (
31). At the counseling session, all participants set a quit date for smoking cessation and discussed a drinking goal, such as abstinence or drinking reduction. The quit date was set to occur within 3 days after completing the varenicline titration schedule to reduce experimental error associated with highly variable quit dates. After randomization, participants attended research sessions at weeks 4, 8, 12, 16, and 26. Each session consisted of research assessments, CO recordings, and, during the 12-week treatment phase, medication pill count. Participants completed brief telephone assessments at weeks 2 and 6 to promote medication adherence and retention in the study.
Assessments
At the in-person intake visit, a series of assessments were conducted for eligibility and individual differences such as demographic characteristics, alcohol use, and tobacco use. These assessments include the Structured Clinical Interview for DSM-5 (
32), the CIWA-Ar, the Fagerström Test for Nicotine Dependence (
33), and the Columbia-Suicide Severity Rating Scale (
34). At intake, all participants were required to have a breath alcohol concentration of 0.00 g/dL and to test negative for all drugs of abuse (except for cannabis). Blood pressure and heart rate were measured at intake and at each visit. Side effects were elicited in open-ended fashion at each study visit and were regularly reviewed by the study physician.
The timeline followback instrument was used to derive the drinking outcomes of interest. The timeline followback consists of a calendar-assisted interview in which participants report on their use of alcohol over the specified assessment period (i.e., typically 4 weeks but up to 10 weeks in the 26-week assessment). A trained interviewer asked participants whether they consumed any alcohol on each calendar day within the assessment period covered by the visit and recorded the number of drinks consumed as standard average drinks (i.e., 12 ounces of regular beer, 5 ounces of wine, 1.5 ounces of distilled spirts). Those drinking estimates were then converted into the desired drinking outcomes over a given assessment period (i.e., baseline, 4, 8, 12, 16, and 26 weeks). The primary outcome of drinks per drinking day is computed by dividing the total number of drinks consumed during the assessment period by the number of drinking days during the same period. The timeline followback has high reliability and is widely used in clinical trials for AUD (
35,
36).
Outcome Measures
For smoking cessation, the a priori outcome was a 7-day point prevalence of nicotine abstinence, bioverified by a breath CO reading ≤5 ppm, which is considered optimal for smoking cessation trials (
37). The primary a priori drinking outcome was drinks per drinking day. The rationale for selecting drinks per drinking day was based on the sample comprising heavy-drinking smokers, for whom the percent of heavy drinking days variable may not be ideal. The heavy-drinking smoker population may not have as many heavy drinking days as samples required to have moderate to severe AUD, which is typical for AUD clinical trials. Hence, drinks per drinking day was selected a priori (i.e., at trial registration) given that it captures reductions in drinking, as opposed to abstinence, yet does not rely on a high prevalence of heavy drinking days. Instead, any reduction in drinking on days on which participants consumed alcohol would be detectable. Secondary drinking outcomes were percent heavy drinking days, number of drinking days, and percent days abstinent. These secondary outcomes were intended to detect additional medication effects and are standard in clinical trials for AUD (
38).
Statistical Analysis
The trial was powered to detect a medium effect size (Cohen’s d=0.5) for between-group differences on the primary outcomes. With >64 participants per group, power to detect such an effect was ≥80% for a two-tailed test and an alpha level of 0.05. Analyses were conducted in SAS, version 9.4 (
39). All analyses were of the intention-to-treat type. Since all participants took the first dose of the study medication under observation during the randomization visit, all participants were included in the subsequent analyses. The dichotomous primary outcome for smoking cessation at the 26-week follow-up was analyzed using a chi-square test comparing smoking cessation rates for the two medication conditions. In these analyses, all individuals who withdrew from the trial were considered to have returned to smoking, per guidelines for smoking cessation trials (
40). Sensitivity analyses were conducted for the smoking cessation outcome, testing alternative assumptions about missing data, as opposed to relying solely on the assumption that data missingness should be scored as a failed cessation attempt (see the
online supplement). For the drinking outcomes, all of which were continuous measures, a series of multilevel models, via PROC MIXED, were conducted for each outcome separately, during the 12-week treatment period and for the entire 26 weeks of the trial, which is consistent with pharmacotherapy trials for excessive drinking (
38,
41). These models tested whether the two medication groups differed in alcohol use over the 12-week medication period and across the entire 26 weeks of the trial. All models for drinking outcomes controlled for baseline drinking levels. Sensitivity analyses were conducted for the drinking outcomes to assess the effects of missing data assumptions (
42) (see the
online supplement).
Results
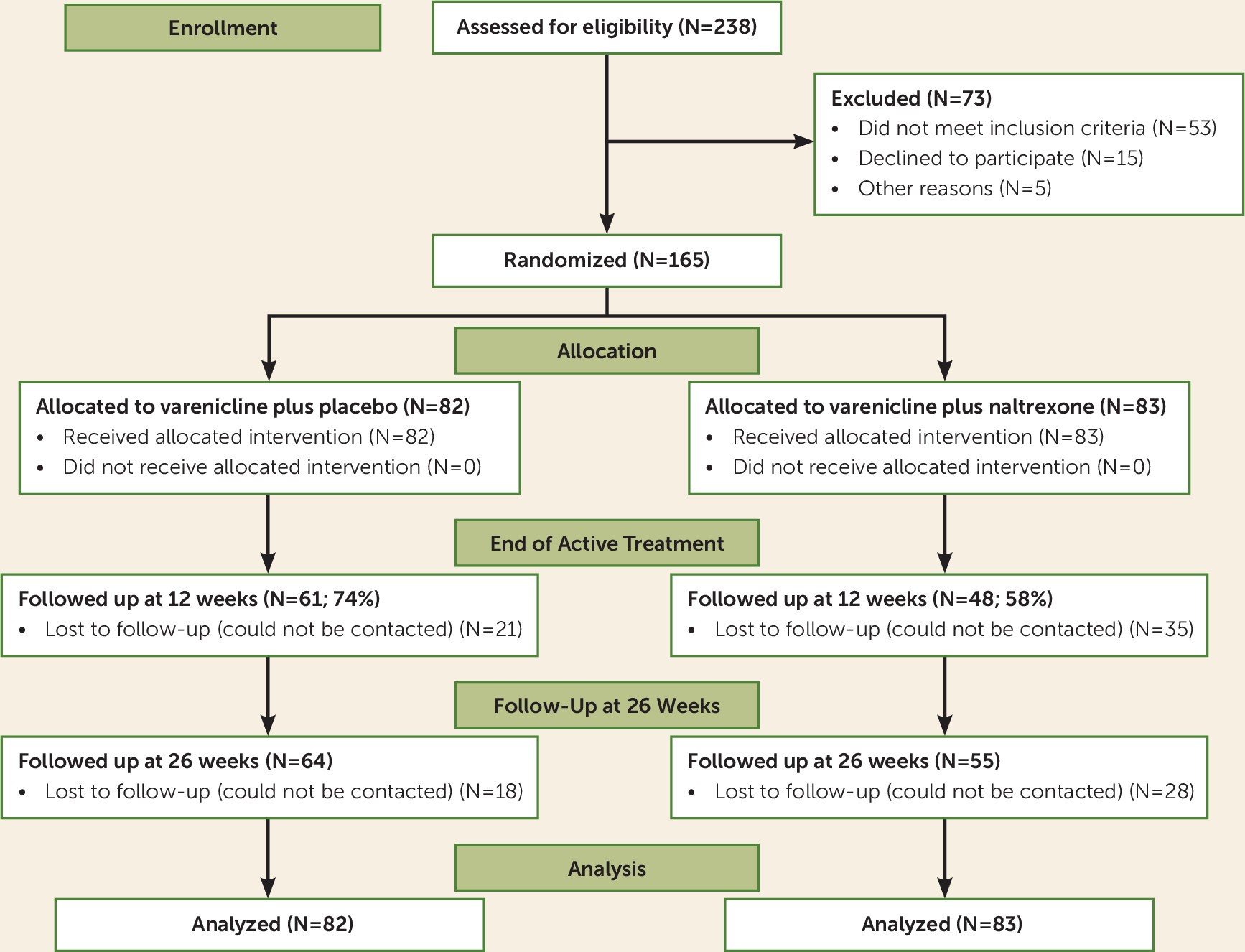
Figure 1 summarizes the recruitment and disposition of the study participants. A total of 165 heavy-drinking smokers underwent randomization, and 119 of them completed the final follow-up at week 26, for a retention rate of 72%. The difference in dropout rates between the two medication groups was not statistically significant (χ
2=2.85, df=1, N=165, p=0.92). Adverse events, recorded in an open-ended fashion at each study visit, are presented in the
online supplement. There were no significant differences in adverse events across the two medication groups across the various symptom categories (p values >0.06). All randomized participants took the first medication dose under observation and were included in the final analysis. Five participants (four male and one female) had reductions in their varenicline dosage, from 2 mg/day to 1 mg/day; all five remained in the study. One participant discontinued the study medications because of adverse events (mainly nausea). The participants’ demographic, smoking, and drinking characteristics are presented in
Table 1; the medication groups did not differ significantly in any baseline characteristic reported in the table.
The overall medication adherence rate across both medication conditions, based on pill count, was 64.12% (SD=0.37). There was a significant effect of medication group on adherence, such that the varenicline plus placebo group had an overall adherence rate of 68.46% and the varenicline plus naltrexone group had an overall adherence rate of 59.83% (F=4.88, df=1, 164, p=0.029), and that is after controlling for the large effect of dropout status on adherence (F=180.61, df=1, 164, p<0.0001). Thus, over and above the effect of medication group on dropout, individuals in the varenicline plus placebo condition had a higher rate of medication adherence, based on pill count, across the 12 weeks of active medication treatment.
Medication Effects on Smoking Cessation
The primary outcome for the trial was 7-day point prevalence of nicotine abstinence, bioverified by a breath CO level of ≤5 ppm at the 6-month follow-up. At the 26-week assessment, of the 165 participants analyzed across both medication conditions, 59 had quit smoking, for an overall quit rate of 35.76%. Analyses within medication groups revealed that in the varenicline plus placebo condition, 37 participants (45.12%) had quit smoking at week 26, whereas 45 participants (54.88%) had not. In the varenicline plus naltrexone condition, 22 participants (26.51%) had quit smoking at week 26, whereas 61 participants (73.49%) had not. The difference in quit rate at the 6-month follow-up between medication conditions was statistically significant (χ
2=6.22, df=1, N=165, p=0.015) and favored the varenicline plus placebo condition (
Figure 2). This pattern of results was also seen at the 12-week assessment, when participants were still on active medication; the quit rate was 53.66% (44/82 participants quit) in the varenicline plus placebo condition, compared with 38.55% (32/83 participants quit) in the varenicline plus naltrexone condition (χ
2=3.79, df=1, N=165, p=0.051) (see Figure S1 in the
online supplement).
To test for sex effects, we ran a multivariate logistic regression, which confirmed the main effect of medication (χ2=6.77, df=1, N=165, p=0.009) and found no effect of sex (p=0.99) or of medication-by-sex interaction (p=0.35) on abstinence at week 26. We also tested AUD severity as a predictor of smoking cessation outcome at 26 weeks and found no significant effect (χ2=1.52, df=1, N=165, p=0.68). Likewise, there was no AUD severity-by-medication effect on smoking cessation outcomes at 26 weeks (Wald χ2=2.99, df=1, N=164, p=0.39). Lastly, sensitivity analyses tested the effects of missing data assumptions—missing at random and missing not at random—on smoking cessation outcomes at week 26; these results are presented in the online supplement. The results of the sensitivity analyses were consistent across different assumptions for missing data, such that significantly higher smoking quit rates were observed in the varenicline plus placebo condition.
Medication Effects on Drinking Outcomes
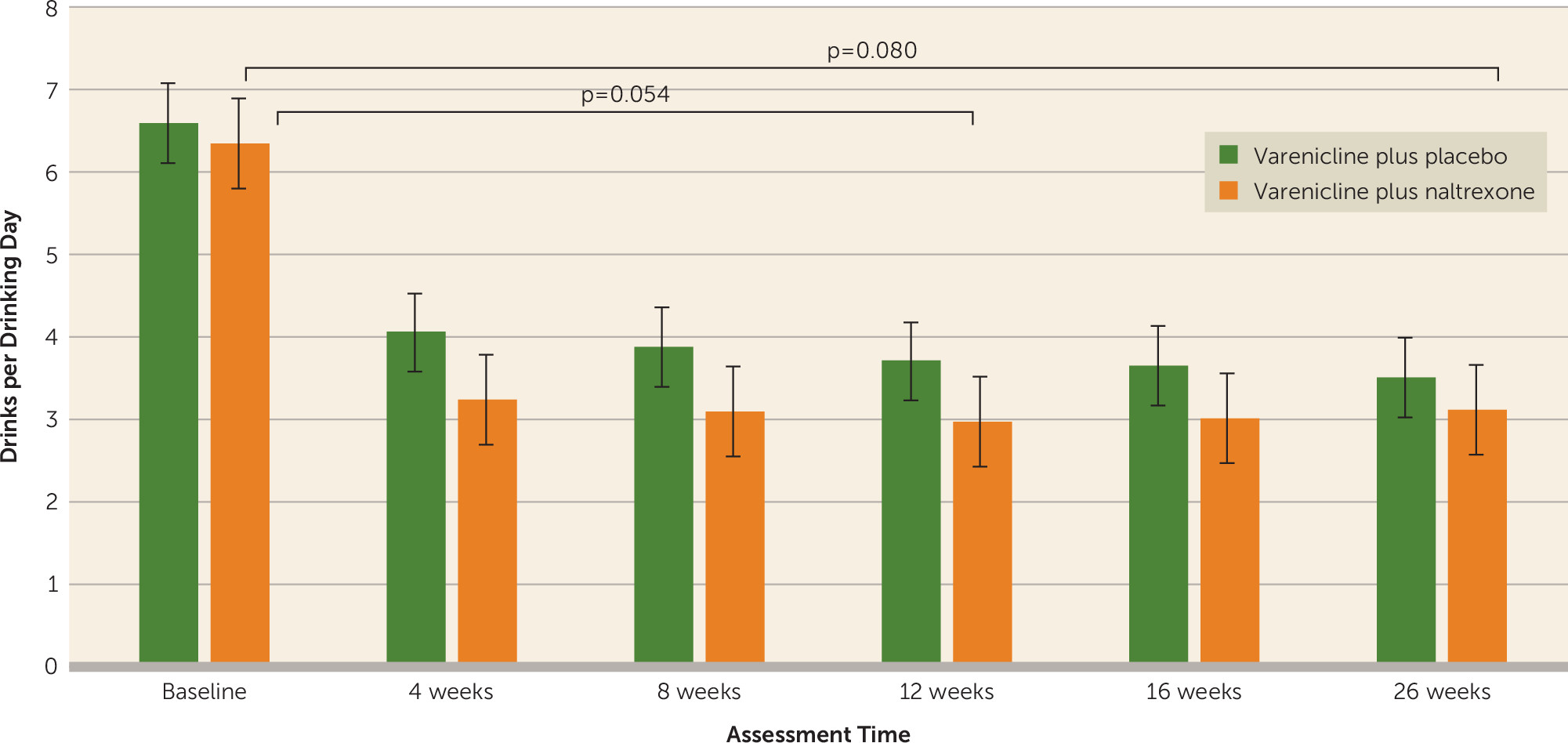
For all drinking outcomes, the course of drinking reduction showed a steep change from baseline to 4 weeks, and these changes were generally maintained at 8, 12, 16, and 26 weeks. Thus, the effect of time on drinking outcomes was captured by three time variables, the first capturing weekly change during the period from baseline to 4 weeks, the second capturing weekly change from 4 to 12 weeks, and the third capturing weekly change from 12 to 26 weeks. Analyses of the primary drinking outcome of drinks per drinking day demonstrated a medication effect, which did not meet the significance threshold, favoring the varenicline plus naltrexone condition across the entire trial, including the active medication period (weeks 4, 8, 12) and the follow-up period (weeks 16 and 26) (β=0.82 SE=0.47, t=1.76, p=0.080). Sensitivity analyses that investigated the influence of different missing data handling assumptions on the findings supported this main effect of medication (see the
online supplement). Analyses also revealed a stronger main effect of medication condition over the course of the 12-week period favoring the combination of varenicline and naltrexone over varenicline plus placebo, although this did not meet the significance threshold (β=0.86, SE=0.44, t=1.94, p=0.054). As shown in
Figure 3, participants in the varenicline plus naltrexone condition consumed approximately 1 less drink per drinking day, on average, during the 12 weeks of medication treatment. This effect was more pronounced in the change from baseline to 4 weeks, when the quit attempt took place. There was a significant effect for the first time variable (baseline to week 4) (average change per week; β=−0.78, SE=0.11, t=−6.89, p<0.0001) but not for the second time variable in the model (weeks 4 to 12) (β=−0.03, SE=0.04, t=−0.76, p=0.45), both of which were entered simultaneously in the single model testing medication effects on drinks per drinking day. Results for the third time variable in the model (weeks 12 to 26) indicated no significant effect (average change per week; β=0.04, SE=0.05, t=0.83, p=0.41). Variance components further indicated that individual change rates exhibited significant variation during the first but not the second or third time periods. There was no medication condition-by-time interaction for any of the three time periods (p=0.34, p=0.50, and p=0.85, respectively). AUD severity was a significant predictor of outcome, with higher severity predicting more drinks per drinking day (p<0.001).
Several variables were used to further probe the main effect of medication on drinks per drinking day. First, regarding the effect of smoking cessation outcome on subsequent drinking in the trial, analyses indicated that the effect of medication on drinks per drinking day was significant (p=0.050) in a model that accounted for whether participants had successfully quit smoking at the 4-week assessment (i.e., first assessment following the planned quit date), but there was no significant medication-by-smoking cessation interaction on drinking outcomes (p=0.47). Second, regarding sex effects, there was a main effect of sex (β=−1.66 SE=0.57, t=−2.90, p=0.04), such that, overall, women reported fewer drinks per drinking day than men at the 4-week follow-up. However, there was not a sex-by-medication (p=0.15) or sex-by-medication-by-time interaction for either the first (p=0.39) or the second (p=0.31) time variable. Third, while AUD severity was a significant covariate in all the drinking outcome models, there was no evidence of a medication-by-AUD severity interaction on drinks per drinking day (p=0.88).
Results of medication effects on secondary drinking outcomes were largely not statistically significant for the time frames of primary interest and are reported in detail in the online supplement. Of note, for percent heavy drinking days across the entire 26 weeks, there was no significant effect of any of the time variables (p values >0.666), nor any time-by-medication interactions (p values >0.619). The overall medication effect was also not significant (β=0.056, SE=0.059, t=0.95, p=0.344).
Discussion
This clinical trial compared the combination of varenicline and naltrexone against varenicline plus placebo for smoking cessation and drinking reduction in heavy-drinking smokers. Based on preliminary studies, we hypothesized that in this superiority trial, the combination of varenicline and naltrexone would be superior to varenicline alone as a smoking cessation aid. Results for smoking cessation were contrary to the initial hypothesis, as we found that varenicline alone led to a 42% quit rate in this sample of heavy-drinking smokers, compared with a 25% quit rate for the combination of varenicline and naltrexone. The observed quit rate for varenicline alone in this sample is highly encouraging, as this is the first large-scale trial of varenicline efficacy focused solely on heavy-drinking smokers. Notably, preliminary findings on varenicline for heavy-drinking smokers have also been positive (
43). If supported by large-scale randomized clinical trials with a placebo arm, varenicline may be especially indicated for smoking cessation among heavy-drinking smokers. In addition, a recent study found that dual nicotine replacement therapy may be as effective as varenicline and have better tolerability (
44), such that comparing varenicline to dual nicotine replacement therapy in heavy-drinking smokers may be warranted.
In essence, we found that there was no benefit of adding naltrexone to varenicline treatment for smoking cessation. In fact, adding naltrexone had a negative effect on smoking cessation rates. Given that in this sample, participants reported a desire to quit smoking and to reduce, as opposed to quitting, drinking, it is possible that naltrexone may work to reduce smoking while not being beneficial to quitting smoking entirely. This is consistent with findings that naltrexone reduced the number of cigarettes per day during the pre-quit phase of smoking cessation (
45) but did not improve smoking abstinence rates (
46) compared with placebo. This is the first trial of the combination of varenicline and naltrexone, and heavy-drinking smokers did worse on the combination treatment than on varenicline alone. Further research exploring the bases for this effect is warranted. In brief, given that varenicline has been found to reduce drinking in trials for AUD (
16,
17), it is possible that its effects on both drinking and smoking present an optimal alternative for this unique subgroup of smokers. Large-scale randomized clinical trials of varenicline compared with placebo are needed to fully ascertain the clinical efficacy of varenicline in heavy-drinking smokers.
Regarding the drinking outcomes in this study, there were significant reductions in drinking across both conditions; however, for our primary outcome of drinks per drinking day, there was evidence for some benefit of combining varenicline and naltrexone, as compared with varenicline alone. This medication-by-time effect approached significance at week 12, and further reduced in significance when extending the analyses to week 26. Analyses of the secondary outcomes did not offer additional support for the benefit of the combination of varenicline and naltrexone for drinking reduction. It was expected that the addition of naltrexone to a varenicline regimen would have unique benefits for drinking outcomes, given its established utility for AUD (
20,
47). Naltrexone is especially useful in reducing heavy drinking among individuals who continue to drink (as opposed to complete abstinence) (
48), and this effect is thought to be achieved via naltrexone-induced blunting of the rewarding effects of alcohol (
49). To that end, this study sample was ideally suited for naltrexone treatment: participants were interested in reducing their drinking, yet their primary reason for seeking treatment was for smoking cessation. Nevertheless, this was a superiority trial, and exceeding the effects of an established pharmacotherapy such as varenicline imposes a high bar for clinical testing. The fact that the combination of varenicline and naltrexone was superior to varenicline alone by showing reductions in drinks per drinking day during the active medication phase suggests that combination pharmacotherapy could be considered in samples of patients with AUD whose primary treatment goal is drinking related. However, it is also notable that the medication effect was reduced at the 26-week follow-up and that the medication effects were approaching significance, particularly during the active medication treatment, at 12 weeks. This effect should be verified in samples of nonsmokers, as recent studies suggest that nicotine use may predict a more positive clinical response to naltrexone (
50,
51).
Another important area in which this study advances the literature is the concurrent treatment of smoking and heavy alcohol use. While the scientific literature largely comprises single-disorder treatment with stringent exclusion criteria (
52), it has been long recognized that co-occurrence and comorbidity are the norm in clinical care settings. Hence, concurrent treatments across disorders holds tremendous promise for patients and health care systems alike (
26,
27). This study suggests that combined outcomes for smoking cessation and drinking reduction are achievable. In reference to the tolerability of both varenicline and naltrexone, this study found no significant differences between the two medication conditions in adverse events reported by participants. Furthermore, this clinical trial was able to recruit a diverse range of participants, with African American being the most common race reported in our sample. Given past difficulties recruiting minorities for participation in clinical trials (
53), recruitment for this study was conducted in a culturally sensitive manner, so as to create an inclusive environment for African American participants (
54) and participants from diverse racial and ethnic backgrounds. As clinical studies are tasked with expediting translation to clinical practice (
55), studying representative samples with co-occurring health conditions may ultimately improve the uptake of science-based treatments in clinical settings.
This study has several limitations that should be considered when interpreting its results. Most notably, in this superiority trial there was not a placebo arm, nor a naltrexone-alone condition. While we believe that varenicline is a first-line treatment for smoking cessation, its efficacy in heavy-drinking smokers should be verified in a placebo-controlled design. This is the first large-scale randomized clinical trial to test the efficacy of varenicline in heavy-drinking smokers, a sizable and treatment-resistant subgroup (
13). Likewise, for the purpose of improving treatment for alcohol-related problems, a comparison of the combination of varenicline and naltrexone against placebo and against naltrexone alone would be ideal in confirming the benefits of this pharmacotherapy combination. More generally, the potential to combine promising pharmacotherapies for AUD with naltrexone has been demonstrated elsewhere and should be carefully considered (
56). An additional limitation was the use of an open-ended adverse event reporting system, which reduced the ability to compare adverse effect profiles to other studies with varenicline and naltrexone that may have used standardized side effect measures. Lastly, retention for this trial reached 72% for the 6-month follow-up, and medication adherence was significantly higher for the monotherapy group. While these are acceptable retention and adherence rates, particularly for a group with co-occurring daily smoking and heavy drinking, combination pharmacotherapy should consider issues of tolerability and adherence. Our preliminary study of the naltrexone and varenicline combination used a lower dosage of naltrexone, at 25 mg/day (
23), and other studies have suggested a potential benefit of lower naltrexone dosages (
57). Previous studies have also found support for the 50 mg/day dosage used in this study. In combination with nicotine replacement therapy, 50 mg/day of naltrexone reduced the likelihood of relapse among participants who smoked during the first week in treatment (
58). Other human laboratory (
59) and clinical trials (
60) have supported the use of 50 mg/day of naltrexone to improve smoking outcomes. On the other hand, studies have found that a naltrexone dosage of 100 mg/day may be necessary to boost the clinical utility of this medication for smoking cessation (
61). It has recently been shown that 100 mg/day of naltrexone may have unique clinical benefits through the blockade of kappa opioid receptors (
62). On balance, further studies seeking to optimize dosage selection may be especially critical in the context of combination pharmacotherapy.