Social Representations in the Medial Prefrontal Cortex
Converging evidence implicates a large-scale cortical network in the two cognitive processes mentioned above: reexperiencing events (REE) and constructive episodic simulation (CES). This circuitry is variably described as the medial network or the larger default-mode network, which includes the medial prefrontal cortex, the hippocampus, and the cingulate cortex, among other areas. Despite some method-based disagreements about its components, all methods place the medial prefrontal cortex in these networks (
32,
33).
In addition to a role in episodic memory, this area supports social cognition by storing a unique form of memory: features of conspecifics and of one’s self. After reviewing the evolution of the medial prefrontal cortex, Murray et al. (
5) proposed that its self-representations endow the medial network with unique properties that characterize human episodic memories, especially a vivid sense of participation in the “here and now.” This proposal drew heavily on the work of Lau and colleagues (
34,
35), who attributed human self-representation to successive rerepresentation of personal actions, intentions, goals, and states, which reside in the medial prefrontal cortex. This region encompasses the cortex extending from the medial frontal pole (area 10) to the caudal boundary of the anterior cingulate cortex (ACC; areas 24, 25, and 32). Comparative neuroanatomy suggests that its rostral, granular component originally evolved in anthropoids and expanded during human evolution (
5). Rostral parts of the medial prefrontal cortex are thus well suited to establish rerepresentations of the “self” by drawing on information encoded in phylogenetically older areas. Rerepresentation involves the abstraction of information by higher-order prefrontal areas from lower-order ones. Rostral, granular prefrontal areas draw on representations in areas situated more caudally, and higher levels of rerepresentation emerged as the granular prefrontal cortex expanded during human evolution. Murray et al. reasoned that if each anthropoid species evolved a modified version of self-representation, suited to the social system of that species, then modern humans also have a species-specific form of self-representation.
Across anthropoids, including humans, the ACC represents social value in addition to other kinds of valuations (
36). Single-neuron activity in the ACC encodes a monkey’s social preferences, acquired through vicarious reinforcement (
37), and selective ACC lesions prevent monkeys from acquiring prosocial preferences (
38). Phylogenetic analysis shows that social complexity increased during the evolution of anthropoids, which descended from primates with solitary social systems (
39). The same analysis reveals that complex social systems evolved convergently in prosimians and anthropoids. So, notwithstanding the social functions of the ACC in other mammals (
40), nonsocial functions of the medial prefrontal cortex were likely co-opted for social ones independently during anthropoid evolution (
41).
In humans, meta-analysis shows that a large part of the medial prefrontal cortex has significant fMRI activations while people make judgments about themselves and others. There are at least two clusters of self-related sites: one in the pregenual cortex (area 32), the other immediately rostral to the first, in the medial frontal pole cortex (area 10) (
42). A meta-analysis of fMRI covariance confirms that these rostral areas process social representations (
43), with three subdivisions: dorsomedial, ventromedial (including subgenual), and pregenual cortex. Performance on both episodic memory tasks and social tasks significantly predicts fMRI activations in all three subdivisions (
43), which points to these areas as key sites for combining the representations of one’s self and events.
REE and CES: Self-Representation and Shared Neural Substrates
From an origin in the medial prefrontal cortex, corticocortical connections can distribute conjunctive self-event representations to other components of the medial network, in which they are thought to infuse episodic memories with a “here-and-now” quality. A subjective sense of REE results: “what it was like” when the event occurred (
5). Self-representations can also establish a sense of participation in events imagined via CES, which generally lacks “here-and-now” qualities. This distinction arises because reality monitoring allows people to differentiate imaginary from actual events, although this function can fail in mental illness (
44).
Episodic memories and imagined events might seem distantly related, but four lines of evidence suggest that the neural networks supporting REE also underlie CES. First, when people either remember events or imagine future ones, overlapping brain regions manifest activations in fMRI studies, implicating a core network in REE and CES (
45,
46). This network includes the medial prefrontal cortex and other medial network areas, such as the hippocampus and retrosplenial cortex (
Figure 2A). Second, remembering and imagining events share properties (
47), such as a positivity bias—that is, a preponderance of memories and imagined events with a positive affective valence. The extent of this positivity bias correlates significantly for reexperiencing past events and imagining future ones (
48). Third, patients with impairments in remembering events also have a poor capacity for imagining future events (
49,
50). And fourth, individual differences in precommissural (but not postcommissural) fornix microstructure correlate with the episodic richness of both past and future autobiographical narratives (
51).
An overlap in the neural circuitry for REE and CES probably reflects their common dependence on mechanisms for mental construction of event sequences and boundaries (
49,
52,
53). Furthermore, memories of past events populate imagined ones.
The medial prefrontal cortex, by rerepresenting one’s self and others at the highest hierarchical level, enables the incorporation of self-representations into event memories, in part via interaction with the hippocampus. The medial prefrontal cortex and the hippocampus play related but specialized roles in this process. Damage to the hippocampus causes an impairment in constructing detailed episodic simulations, but patients with such damage incorporate themselves normally. Patients with medial prefrontal damage show the opposite pattern; they can construct detailed mental simulations but rarely incorporate themselves (
54).
Emotion and the Amygdala
Emotional responses are prominent features of both REE and CES, an observation with several clinical implications. Research on macaques reveals important circuitry underlying emotional features of REE and CES in humans.
Specifically, this work shows that the medial prefrontal cortex (
Figure 3) and the hippocampus have dense interconnections with the amygdala. According to Price and Drevets (
55), the amygdala and medial prefrontal cortex are reciprocally connected, especially the latter’s caudal parts. The hippocampus and the amygdala also have dense interconnections with each other, mostly involving the temporal (anterior) hippocampus (
56,
57), which underlies mental scene construction (
58). The hippocampus also sends dense inputs to the medial prefrontal cortex, which, like amygdala inputs, concentrate in its caudal aspects (
59). Thus, inputs from the amygdala and hippocampus overlap in the medial prefrontal cortex, although they terminate mostly in different laminae (
60). Through these inputs and local circuitry, the medial prefrontal cortex has the connections needed to integrate information from the amygdala and hippocampus with representations of one’s self.
The understanding of neuroanatomy in this rich detail depends on research in macaque monkeys. These species and other anthropoids can provide this information for a simple reason: they have a homologue of the granular prefrontal cortex, but other common laboratory animals, such as rats and mice, do not (
5,
29). For this reason, insights about brain structures conserved among anthropoids can, by applying an evolutionary perspective, contribute to understanding of human-specific cognitive functions and their relations with mental illness.
Several findings have implicated these connections in memories of emotional events, whether imagined or reexperienced. For example, neuroimaging studies have identified regions with greater activation for emotional events than for neutral events. Two meta-analyses using this approach have implicated the amygdala and hippocampus, along with the entorhinal, perirhinal, and visual cortex, in the encoding and retrieval of emotional episodic memories (
61,
62). Another fMRI study found that functional connectivity between the amygdala and hippocampus increased during the retrieval of emotional contextual information (
63).
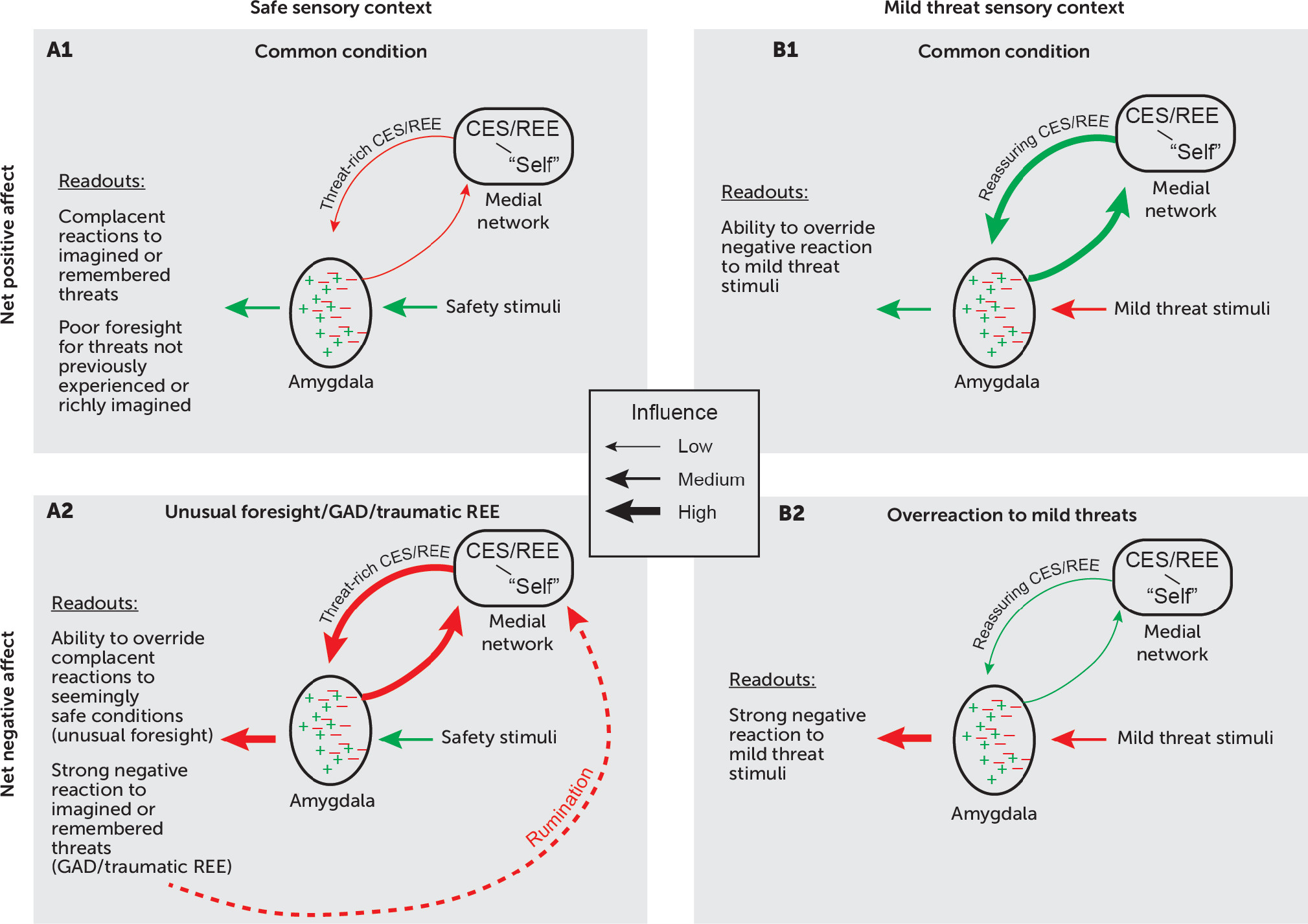
Figure 4 presents a conceptual model of the amygdala’s hippocampal and medial prefrontal inputs, which contribute to emotional responses during REE and CES. These inputs combine with those from sensory (and other) areas of the cortex to regulate the amygdala’s net output. Reciprocal projections from the amygdala amplify these responses. Before discussing this model, we highlight some current ideas about amygdala function.
It is generally accepted that the amygdala biases behavioral outputs to enhance Darwinian fitness. This function involves exploiting resources (e.g., nutrients, fluids, warmth), avoiding predators, producing progeny, and learning about sensory cues that signal resources, threats, or safety (
56). It is crucial that such biases adapt to an individual’s current state and motivations, and experiments on both macaque monkeys and rodents have shown that the amygdala plays a necessary role in value updating based on current biological needs (
64).
Two widespread misunderstandings have hampered attempts to reach consensus concerning amygdala function in this context. First, some authors have argued that the amygdala functions primarily, if not exclusively, in threat processing and negative affect (
65). The vast literature on fear conditioning has contributed to this misconception, and many discussions of amygdala function continue to focus exclusively on negative affect. However, experimental evidence from both rodents and macaques has overturned this idea conclusively. Holland and Gallagher (
66) reviewed evidence from several laboratories showing that the rat amygdala plays an important role in reward processing and positive affect, and Janak and Tye (
67) reached the same conclusion more recently. And in macaque monkeys, the amygdala plays a necessary role in updating valuations that have a positive affective valence (
68,
69).
Second, the idea that the prefrontal cortex predominantly “inhibits” amygdala output is contradicted by neuroanatomical evidence from macaques. These studies show that cortical projections to the amygdala, which are excitatory, terminate on both excitatory and inhibitory interneurons (
70–
72). Thus, cortical influences on the amygdala can modulate its outputs both positively and negatively, depending on the precise circuitry engaged.
Figure 4 depicts this idea with green symbols for circuits contributing to positive-valence emotional readouts and red symbols for negative-valence readouts. In both cases, inputs from the medial prefrontal cortex and the hippocampus engage amygdala circuits that influence emotional responses to both remembered and imagined events. Inputs from sensory areas of the cortex, including homotypical association areas such as the inferior temporal cortex, provide current sensory contexts.
In
Figure 4A2, inputs to the amygdala from both the hippocampus and medial prefrontal cortex convey threat-rich signals generated via retrieved episodic memories or by CES, which strongly activates threat-avoidance circuits via the amygdala. According to this model, when threat-rich signals from the medial network override signals reflecting a safe sensory context, a negative affective state results. For traumatic REE, this means that after a trigger prompts the recall of a harrowing event, a currently safe sensory context does little to mitigate a patient’s emotional responses. For CES, a powerful emotional reaction occurs for imagined harmful events despite concurrent safety signals. Most research on CES stems from studies of episodic memory, but CES also is highly relevant for studies of anxiety because overactive CES represents a cardinal feature of generalized anxiety disorder, an illness defined by excessive worry. In both generalized anxiety disorder and worries that occur in other mental disorders, maladaptive feedback loops (dashed red line in
Figure 4A2) can arise, in which rumination about future harms and failures generates persistent anxiety.
In
Figure 4B2, a negative affective state prevails for a different reason. Mild threats should cause little anxiety, if any, and in most people they do not. However, if reassuring REE or CES have insufficient influence over the amygdala’s output, a negative affective state will predominate despite a low or nonexistent risk.
Note that
Figure 4A1 and 4B1 do not necessarily reflect optimal emotional responses. Some people respond inadequately to threats they have not experienced or are incapable of imagining in rich, predictive detail (
Figure 4A1). In contrast, individuals with a highly cultivated capacity for CES (unusual foresight) can act in accordance with their imagination rather than their experience, thereby avoiding danger (
Figure 4A2). Of course, excessive imagination of risks can create problems of its own.