Harmful use of alcohol causes more than 5% of the disease burden worldwide (
1), but a great proportion of individuals with alcohol use disorder (AUD) do not respond to currently available pharmacological and behavioral treatments, with more than 70% of those entering treatment relapsing within 1 year (
2). The
N-methyl-
d-aspartate receptor antagonist ketamine is a promising candidate therapy in AUD for several reasons. First, substantial evidence supports the antidepressant properties of subanesthetic doses of ketamine (
3), leading to the recent U.S. Food and Drug Administration and European Medicines Agency approval of esketamine, an enantiomer of ketamine, for use in treatment-resistant depression. Depressive symptoms are common in individuals entering treatment for AUD, and the likelihood of alcohol relapse is elevated in patients with such symptoms (
4,
5). Ketamine may support alcohol abstinence by temporarily alleviating depressive symptoms during the high-risk relapse period in the weeks after detoxification.
Second, ketamine might aid alcohol abstinence by providing a window during which psychological therapies can be more effective. Evidence from preclinical studies suggests that ketamine increases synaptogenesis and neurogenesis, known to be disrupted with addiction (
6,
7). Learning and planning are impaired in patients with AUD, and these deficits likely underpin the limited effectiveness of therapy in patients suffering from AUD (
8,
9). Ketamine may provide a temporary boost to synaptogenesis and neurogenesis, which may allow psychological therapies and new strategies for managing addiction to embed more readily (
10). There is little empirical evidence on the effectiveness of psychological therapy provided in conjunction with ketamine treatment, but one study suggested that 10 weeks of cognitive-behavioral therapy (CBT) alongside ketamine infusions may prolong ketamine-induced symptom reduction in treatment-resistant depression (
11). The subjective experiences that accompany ketamine infusions may provide a new perspective that may be helpful in psychological therapy. Ketamine induces a dose-dependent sense of dissociation and disembodiment that has been described as facilitating an “observer state” similar to that described in mindfulness, which may be helpful for allowing patients to consider thoughts and emotions from a more removed perspective (
12).
Third, several studies have directly investigated the effect of ketamine on patients with problematic alcohol use. An early, nonrandomized study (
13) found that three subanesthetic doses of ketamine (2.5 mg/kg i.m.) adjunctive to psychodynamic psychotherapy led to a 1-year abstinence rate (at outpatient follow-up) of 66% in a group of inpatients with AUD after detoxification, compared with 24% in a conventional-treatment control group. The positive impact of ketamine on AUD was corroborated recently (
12) in a study of 40 outpatients randomized to a single infusion of either ketamine (0.71 mg/kg i.v.) or the active placebo midazolam alongside motivational enhancement therapy in both conditions. At 21 days, 47% of the ketamine group reported using alcohol, compared with 59% of the midazolam group. In a study of individuals with hazardous drinking patterns (
14), one intravenous ketamine infusion combined with a memory reactivation protocol, but no therapy intervention, was associated with reduced alcohol use at 6 months.
Given the antidepressant properties of ketamine, very early evidence that it might aid psychological therapy, and a few studies showing initial benefits of ketamine as a treatment for AUD, in the present study we set out to investigate the safety and feasibility of ketamine infusions compared with saline infusions in increasing abstinence in patients with alcohol use disorder. In this study, three ketamine infusions were administered weekly, as this has been shown to be effective in earlier research (
13). We furthermore aimed to pilot ketamine combined with mindfulness-based relapse prevention therapy (henceforth “therapy”) compared with ketamine plus alcohol education (as a therapy control). This type of psychological therapy was chosen because it has been shown to be effective, and the ketamine experience can be considered to potentially promote engagement in mindfulness practice by giving experiential insights. Thus, in this phase 2 clinical trial, we compared four treatment conditions: 1) ketamine (active) and therapy (active), 2) ketamine (active) and alcohol education (control), 3) saline (control) and therapy (active), and 4) saline (control) and alcohol education (control). We hypothesized that ketamine plus therapy (active plus active) would be most effective in sustaining abstinence and that the lowest abstinence rates would be in the placebo plus education group (control plus control).
METHODS
Participants
Participants were recruited from the community through social media and newspaper and radio advertisements as well as from primary care and secondary care drug and alcohol services.
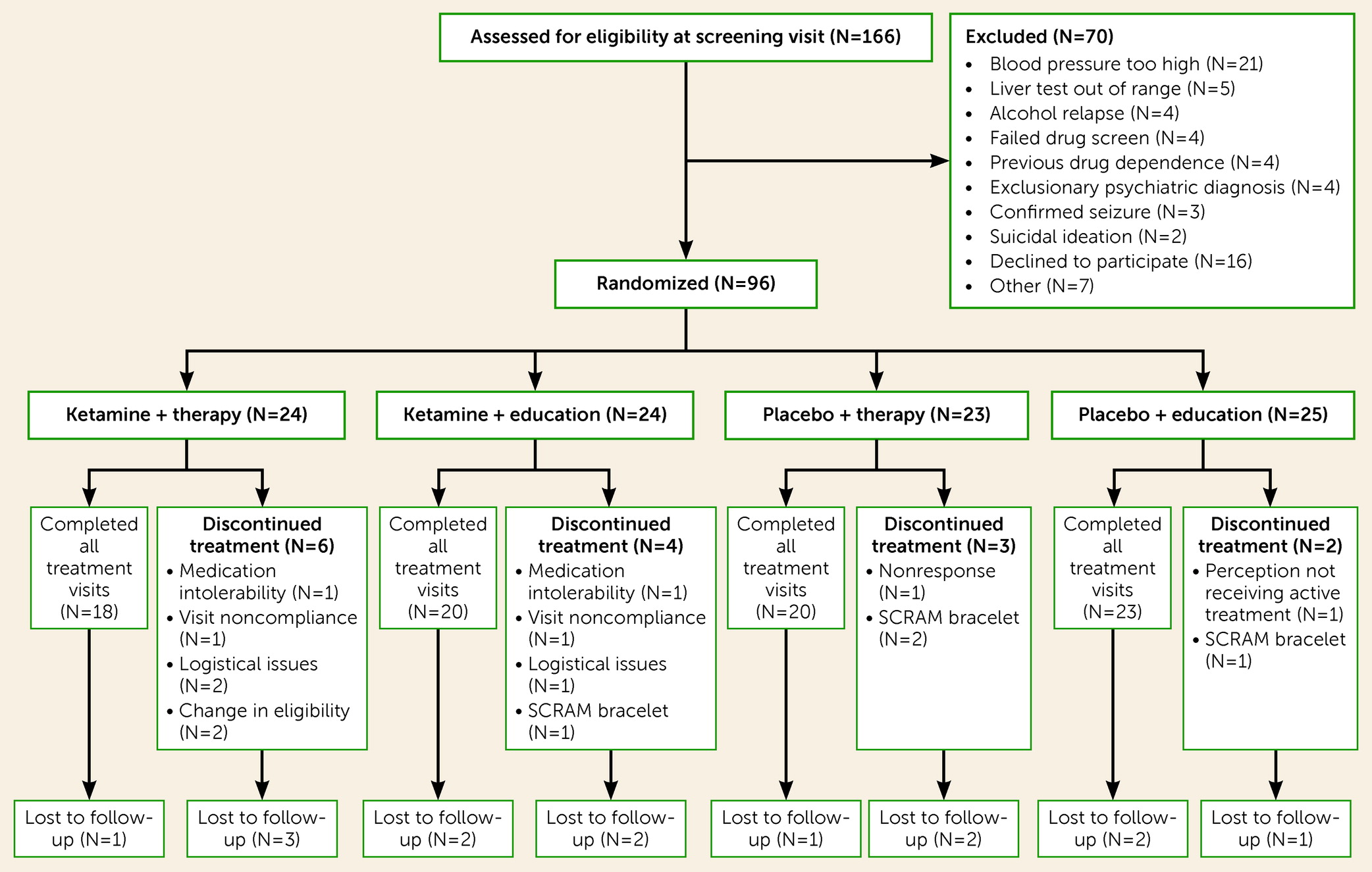
All participants had to achieve initial abstinence at randomization, meaning that they had to be abstinent for at least 24 hours and have a reading of 0.0 on a breath alcohol test at the baseline visit. This allowed us to investigate the impact of ketamine on prolonging abstinence. Participants were also required to have the goal of abstinence for at least the 6 months of the trial. Current level of alcohol use was assessed during an initial telephone screening, and alcohol-abstinent individuals were immediately invited to a screening visit. Individuals who were drinking at levels that meant they could safely cut down to abstinence within 4 weeks were asked to do so and then scheduled for a screening visit. Alternatively, potential participants were encouraged to undergo a supervised detoxification in primary care or through their current treatment provider, and once initial abstinence had been achieved, they were invited for a screening visit. At the screening visit, after written informed consent was obtained, eligibility was determined by the study physician, based on the patient’s medical history, physical examination, mental health assessments, blood and urine analysis, and a breath alcohol test. At the end of the study, participants were remunerated to compensate them for the time spent in the study, at a level correspondent to the national living wage.
Eligible participants had to be 18–65 years old, meet DSM-5 criteria for moderate to severe AUD or DSM-IV criteria for AUD, have a good command of the English language, be currently abstinent from alcohol, and have a negative urine screen for all drugs apart from cannabis and benzodiazepines; these exceptions were based on the long half-life of these drugs and the fact that cannabis is comorbid with AUD and benzodiazepines are commonly prescribed in AUD for sleeping problems. Current or past dependence on either of these drugs was an exclusion criterion.
Key exclusion criteria were uncontrolled hypertension (systolic blood pressure ≥140 mmHg and diastolic blood pressure >90 mmHg), the use of antihypertensives or antidepressants, current suicidal ideation, a diagnosis of any current or past psychiatric disorder (except for depression, anxiety, AUD, or alcohol dependence) or of substance dependence (except for AUD) or ever seeking professional help for dependence on an illicit substance. Study applicants who had more than 10 previous inpatient alcohol detoxifications or a history of harmful ketamine use were also excluded (a full list of the inclusion and exclusion criteria is provided in the
online supplement).
All procedures and patient visits took place at either the National Institute for Health Research (NIHR) Exeter Clinical Research Facility or the NIHR University College London Hospitals Clinical Research Facility. The trial was registered at
ClinicalTrials.gov (NCT02649231) and EudraCT (2015-000222-11) (
15). Ethical approval was granted by the South West–Central Bristol Research Ethics Committee (reference number 15/SW/0312) and the Medicines and Healthcare Products Regulatory Agency. All analyses were preplanned and registered at EudraCT and
ClinicalTrials.gov unless indicated otherwise.
Study Design
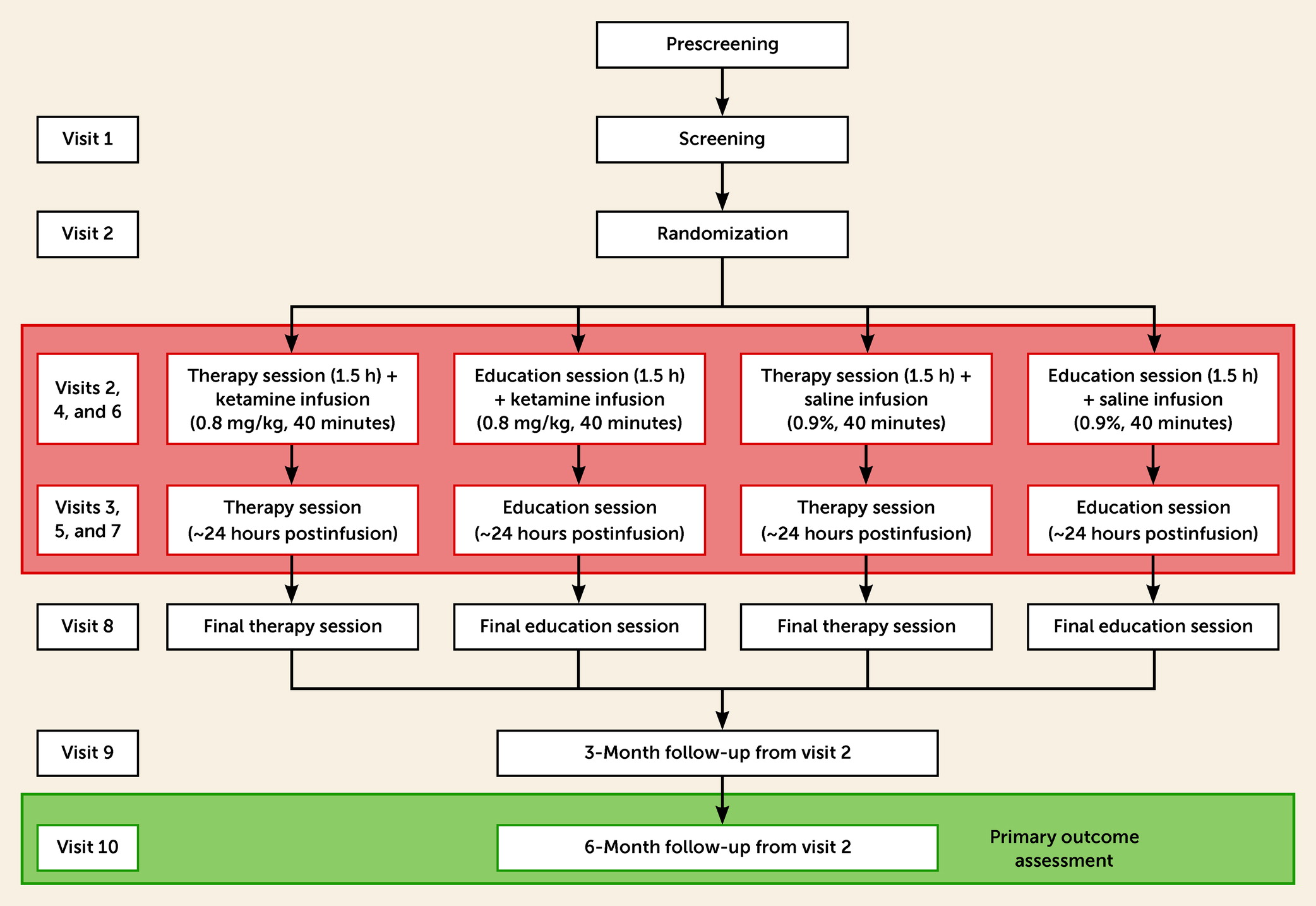
In this double-blind phase 2 clinical trial, recently detoxified adults with AUD were randomly assigned to one of four treatment arms: 1) ketamine (active) and therapy (active), 2) ketamine (active) and alcohol education (control), 3) saline (control) and therapy (active) and, 4) saline (control) and alcohol education (control). Participants were invited to attend 10 study visits (
Figure 1). Self-reported drinking events were recorded at every visit using the timeline followback method. Participants were provided with an alcohol diary to record their alcohol use between visits 8 and 9 and between visits 9 and 10. A Secure Continuous Remote Alcohol Monitor (SCRAM; Alcohol Monitoring Systems, Inc.) bracelet for continuous alcohol monitoring (every 30 minutes) was attached before randomization at visit 1 or 2 and removed at visit 8 (end of treatment), to corroborate self-reported alcohol timeline followback outcomes.
Randomization and Masking
Participants were randomized in a 1:1:1:1 ratio using a block design stratified by treatment site to one of the four treatment arms at the beginning of visit 2. All staff except for pharmacy staff, who had no contact with participants, were blinded to drug treatment allocation, and all except the therapists were blind to the allocation to therapy or education.
Therapy and Alcohol Education Control
At visit 2 and the subsequent six visits, participants received either manualized therapy or alcohol education as a placebo control for therapy. Both were administered by trained psychologists, with all therapists delivering both types of treatment. The sessions were timed so that the infusion was always preceded by a therapy or alcohol education session and followed by another therapy or alcohol education session about 24 hours later.
Therapy.
The aim of the seven therapy sessions based on manualized mindfulness-based relapse prevention was to support the participants in developing an enjoyable and meaningful life without alcohol (
16). Each session was designed to last 1.5 hours and contained one topic related to each of the two overarching themes of the therapy: relapse prevention and the promotion of well-being. In between these two main themes, a different relaxation or mindfulness exercise was introduced in each session. The sessions covered a range of relapse prevention techniques, including dealing with high-risk situations, activity scheduling, and problem solving, alongside dealing with thinking biases (CBT-based), mindfulness practice, and techniques such as urge surfing. Patients were also required to reflect on resources needed for a meaningful life without alcohol. Between sessions, patients used journals to record and reflect on their experiences and completed a number of exercises, alongside mindfulness practice. All therapy sessions were recorded, and an independent consultant clinical psychologist reviewed recordings on a weekly or biweekly basis to check adherence to the treatment protocol. The therapy manual was incorporated into a step-by-step scripted “guidebook” for the participant and therapist that was designed to be prescriptive, to facilitate adherence to the therapy protocol.
Alcohol education.
The seven alcohol education sessions were also designed to last 1.5 hours so that interpersonal interaction time matched the psychological therapy, to act as a control for the therapy condition. During these sessions, the focus was on educational topics, including the driving forces of addiction, the biological effects of alcohol, and ways to improve healthy living and nutrition. In contrast to the psychological therapy, these sessions had no formal psychological components relating to personal relapse prevention strategies, mindfulness, or the promotion of personal well-being.
Drug Administration
The infusions were administered at visits 2, 4, and 6. These visits were spaced apart a minimum of 1 week and a maximum of 3 weeks and lasted for 40 minutes. Ketamine (0.8 mg/kg) and placebo (0.9% saline) of the same volume were administered as intravenous infusions. The dose was higher than in depression studies, based on findings of possible cross-tolerance to ketamine in people with AUD (
17), and it roughly equates to what was suggested to be the lowest effective intramuscular dose (1.2 mg/kg) in alcohol-dependent patients (E. Krupitsky, personal communication, 2012). The intravenous route was used because it is considered the best method to control ketamine blood levels and is associated with fewer adverse effects, such as nausea, than intramuscular administration. Intravenous administration has by now been established as the conventional method for administering ketamine for therapeutic purposes. Saline was used instead of an active placebo because upon starting this study, it was the first in this patient group since the early work in Russia (
13), and we were concerned that an active placebo (e.g., a benzodiazepine) might have unintended therapeutic consequences (
18).
Before each infusion, patients were prepared in terms of potential ketamine experiences by the therapist (see reference
19 for further details) and how they might reflect on the previous therapy session during the drug experience, including directions to use the relaxation or mindfulness techniques learned prior to the infusion during the experience. Patients were asked to bring to mind, where possible, their intention for a life without alcohol. A therapist was present and available throughout the infusion to provide reassurance if the patient required it.
The infusion was administered by an anesthetist through a cannula in a vein in the antecubital fossa. Blood pressure, heart rate, and blood oxygen saturation levels were measured. A psychologist and a nurse were present during the infusion. During the infusion, participants listened to instrumental music through headphones in a single-bed hospital room to facilitate relaxation and minimize distraction from external stimuli. Participants rated potential side effects at −20 minutes, 0 minutes (start of infusion), 20 minutes (midinfusion), 40 minutes (end of infusion), and 60, 80, 100, and 120 minutes after the infusion. These were assessed by a research nurse or psychologist.
Primary Outcomes
The co–primary outcomes were self-reported percentage days abstinent and confirmed alcohol relapse at 6 months after first infusion, both measured using the Alcohol Timeline Followback self-report questionnaire. Confirmed relapse for this study was defined as one or more days of heavy alcohol use; heavy use was defined as >64.8 g of pure alcohol for men (8.1 standard U.K. units) and >52.0 g for women (6.5 standard U.K. units) per day (
20). Abstinence was defined as no alcohol consumption.
Secondary Outcomes
Alcohol-related secondary outcomes were self-reported relapse and percentage days abstinent at 3 months. Other secondary outcomes included depressive symptoms, measured using the Beck Depression Inventory (BDI) (
21) and the Hamilton Depression Rating Scale (HAM-D) (
22); general health, measured by the 12-item Short Form Survey (SF-12) (
23); psychotomimetic experiences (assessed before drug administration and included to index any protracted psychosis-like effects of ketamine and not as an indicator of acute effects), measured by the Psychotomimetic States Inventory (
24); level of cigarette dependence, measured by the Fagerström Test for Nicotine Dependence (
25); alcohol craving, measured by the Alcohol Craving Questionnaire (
26); and SCRAM bracelet alcohol readings. The assessment time points for each measure as well as for other measures not presented here are listed in the
online supplement.
Subjective Drug Effects
Other safety measures included acute subjective effects of ketamine assessed by the researcher through a visual analogue scale of common ketamine effects, vital signs, alcohol breath monitoring, and laboratory tests of liver function and ketamine as well as urine screens for pregnancy and drug use (
27).
Blood Sample Analysis
Ketamine blood concentration was measured at each postrandomization visit and twice on infusion visits: shortly before and 2 hours after infusion.
Statistical Analysis
The main analytic method for all analyses was intention-to-treat (participants were analyzed according to their treatment allocation) and we used observed data only. All inferential analyses (for both primary and secondary outcomes) included adjustment for treatment site. For the primary outcomes, further sensitivity analyses were performed, including imputation of missing data and participants who received the full treatment. The study was not powered to assess an interaction between the drug and the therapy condition.
Self-reported alcohol relapse status and percentage days abstinent from randomization to 6-month follow-up were reported descriptively by treatment arm. Only participants with a minimum of 159 days of completed drinking self-report data were included in the main intention-to-treat analysis of alcohol relapse status, as this was the shortest duration of time before any participant completed the 6-month follow-up (23–25 weeks) in the study. Reporting time was capped at 180 days, but further sensitivity analyses were conducted with imputed data (multiple imputation method) and a per protocol analysis of only participants who received the full treatment. Logistic regression modeling was used to compare the ketamine group with the placebo group (combined across therapy and alcohol education). Additional models compared ketamine plus therapy and ketamine plus education, and ketamine plus therapy and placebo plus alcohol education. Self-reported percentage days abstinent at 3 months and longest abstinent spell within 3 months were also reported descriptively and analyzed using linear regression modeling, with the only sensitivity analysis being adjustment for baseline alcohol use.
Other secondary outcomes were reported descriptively at baseline, 3 months, and 6 months. Inferential analyses using linear regression with adjustment for site and baseline scores were used to compare the combined ketamine group and the combined placebo group at 3 months and 6 months. Repeated-measures analyses using hierarchical linear modeling with a random effect on participant were used to investigate the effects of ketamine compared with placebo for questionnaire outcomes across baseline, 12 days, 3 months, and 6 months, including all participants with data for at least one of these time points. Analyses for the Fagerström Test for Nicotine Dependence included only participants who were smokers at baseline.
For continuous data, effect sizes were calculated as standardized mean differences with associated 95% confidence intervals. If confidence intervals cross zero, this can be interpreted as a nonsignificant effect (alpha=0.05). The size of the value indicates the magnitude of the difference (
28). For dichotomous data, odds ratios were calculated, which can be interpreted as percentage reduction if negative, and percentage increase if positive. If confidence intervals do not include 1, then this can be interpreted as a significant difference (alpha=0.05).
An exploratory analysis was conducted that was not in the original statistical analysis plan: The interaction between the ketamine and therapy conditions on percentage days abstinent at 3 and 6 months was tested using logistic regression modeling in the intention-to-treat population.
All analyses were performed using Stata, version 16. The statistician was blind to treatment group for the analyses of the primary outcomes and alcohol-related secondary outcomes.
DISCUSSION
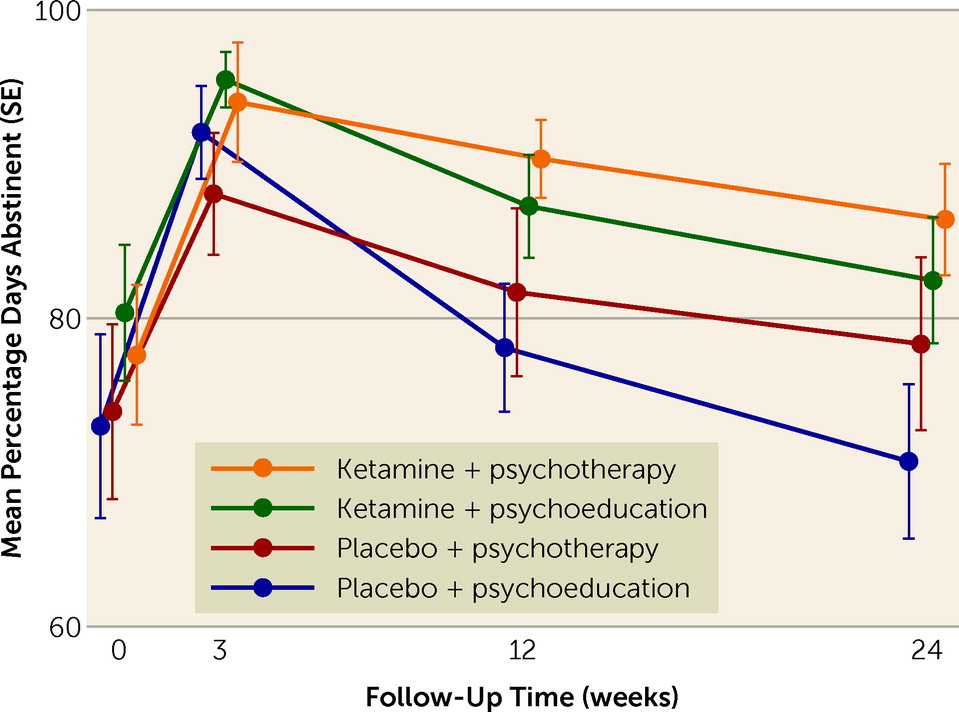
In this proof-of-concept study, we set out to examine the effect of ketamine alongside manualized mindfulness-based relapse prevention therapy on alcohol intake and relapse in currently abstinent patients with AUD over 6 months. The results showed that ketamine increased the number of days abstinent from alcohol at 3 and 6 months compared with placebo. The greatest difference in percentage of days abstinent from alcohol was between patients given ketamine and therapy and those given placebo and education. Overall relapse did not differ significantly between groups.
The longevity of the effect on percentage days abstinent was impressive, being maintained at 6 months following entry into the study after only three infusions. To our knowledge, this is the first phase 2 clinical trial to examine the therapeutic effects of ketamine in addiction over this long a follow-up period. The long-lasting nature of the therapeutic effect we saw here for alcohol use is consistent with other research in groups with AUD (
12) but contrasts with studies in depression, where changes in symptoms are maintained for only around 2 weeks following infusion (
29). The overall beneficial effect of alcohol abstinence and the participants’ adherence to the abstinence protocol were confirmed by the observation that liver function improved over the course of the trial. The impact of ketamine on alcohol abstinence was evident only for percentage days abstinent, not for relapse, which may be because binary outcome variables are less sensitive to detecting differences than more granular, continuous variables. Further, participants were required to have the goal of abstinence for at least the 6 months of the trial, but total abstinence was not a long-term goal in some cases, and this may have affected the findings. Nonetheless, the reductions in drinking and the concurrent improvements in liver function are clinically important, as these changes represent reductions in both mortality and morbidity in this often difficult-to-treat group.
To our knowledge, this is the first study in clinical research to include ketamine combined with psychological therapy alongside ketamine combined with a comparison “psychological” placebo. Alcohol education was used here as a therapy control, and it was associated with a smaller effect on drinking compared with the mindfulness-based relapse prevention therapy. While the sample size was small, these data suggest a possible beneficial effect of combining ketamine and psychological therapy that warrants further investigation. Whereas in the early work in AUD by Krupitsky and colleagues (
13), ketamine was given alongside psychotherapy, in the contemporary research on treatment approaches in depression, ketamine has largely been given alone. Our findings and other emerging data (
11) tentatively suggest that adding therapy may be a fruitful avenue for prolonging the clinical benefits of ketamine in both substance use disorders and depression. Recently Dakwar and colleagues combined ketamine with motivational enhancement therapy for AUD (
12). Based on ketamine’s demonstrated positive effects on motivation to quit cocaine (
30), combining these two interventions was expected to increase motivation to achieve and maintain alcohol abstinence. The Dakwar et al. study and the present study demonstrate mindfulness-based approaches to be effective in substance use disorders. Intuitively, this therapeutic approach is a good fit with ketamine, where the drug experience can act to bridge and bring added insights to early-stage mindfulness practice (
31). The original work by Krupitsky et al. (
13) used transpersonal therapy approaches incorporating elements of aversive therapy to facilitate aversion toward alcohol; this seemed to produce more pronounced effects, although the studies were conducted under very different conditions compared with more recent work. Participants were recruited from Russian alcohol and drug inpatient treatment settings. The dose and administration route in the present study also differed from those in the earlier research, where a single, higher dose of ketamine (2.5–3 mg/kg) was administered intramuscularly (
13,
32); intramuscular administration was chosen in the earlier studies because of its longer-acting acute effects compared with intravenous administration. Concomitant medications were also given in the earlier studies (aethimizole, bemegride) to attempt to counter the amnestic effects of ketamine. The dose and administration route in the present study resemble those in Dakwar and colleagues’ recent randomized controlled trial for AUD (
12), although in that study a single dose (0.7 mg/kg i.v.) was given to people meeting criteria for mild AUD who were currently drinking. The present study adds to the literature by demonstrating that repeated doses of ketamine are safe and efficacious in prolonging abstinence from alcohol in people with severe AUD who had stopped drinking prior to treatment. Dose-ranging studies have not been conducted, but it is important to establish the minimum effective dose, as ketamine treatment studies in AUD have generally opted for higher doses than those used in treatment-resistant depression. Future work should consider conducting dose-ranging studies.
An effect of ketamine on depressive symptoms at 3 months was found when assessed with the self-rated BDI, but not the clinician-rated HAM-D. Generally, the HAM-D is believed to place emphasis on somatic symptoms, whereas the BDI focuses on depressive cognitions (
32,
33). It should also be noted that depression scores in this sample were on average low, likely as a result of the use of antidepressants being an exclusion criterion. Caution is therefore warranted for any interpretation of changes in depressive symptoms. One explanation for our findings might be that ketamine specifically affects anhedonia (
34), as in this study we found anhedonia to be reduced at 3 months as assessed by the Psychotomimetic States Inventory anhedonia subscale, consistent with research in depression.
That ketamine can reduce both alcohol use and depression in AUD is encouraging therapeutically. While a clear link between depression and AUD is acknowledged, alcohol and mental health services still struggle to meet the needs of dual-diagnosis patients (
35), so ketamine may represent a solution to this long-standing comorbidity. Identifying transdiagnostic factors common across depression and substance use disorders that may be common targets for ketamine—for example, alterations in reward sensitivity and anhedonia—will be important in advancing the use of ketamine in dual-diagnosis patients.
There were no serious adverse events associated with the trial drug, and adverse events were generally mild, suggesting that this treatment is well tolerated in this population. Ketamine in anesthesia is indicated for use with caution in people with AUD in the summary of product characteristics, although the results of this study suggest that at a subanesthetic dose, it is a well-tolerated treatment in this group, and that concurrent alcohol use problems need not be an exclusion criterion for ketamine treatment in other psychiatric settings, such as depression (
36).
This study had a number of limitations, notably that the generalizability of the study findings is limited by the rigid enrollment criteria, such as the prohibition of antidepressant use. Furthermore, the blinding of both study conditions (psychotherapy and ketamine) was challenging, especially if participants had prior experience with either ketamine or psychological therapy. Saline was used instead of an active placebo, but studies reported since the start of our own study have demonstrated that midazolam does not have unintended treatment consequences and indeed is associated with reduced engagement with treatment in this patient group (
12). The challenge of blinding to ketamine effects is a limitation of the study, and although it would not be entirely circumvented by use of midazolam as a control, particularly in a group of patients who are not naive to benzodiazepines or ketamine, it would certainly be reduced. Around one-third of patients in our placebo group believed they had been given the active drug, whereas nearly all the patients in the ketamine group thought they had been given the active drug, which could have an impact on their self-perceived efficacy in alcohol use. Therefore, future studies should use an active placebo to better maintain the blind. Given the functional unblinding component associated with ketamine, future studies should systematically ensure that all assessments are conducted by a person who has not observed any part of the drug treatment.
The inclusion in our sample of some individuals who had prior experience of ketamine may have compounded functional unblinding issues. Individuals with more positive expectations of ketamine based on previous experiences may have been more likely to volunteer to take part in the trial, although the majority of participants (73%) reported no previous ketamine use. While including this group may be seen as a weakness of the study, the absence of subsequent problematic ketamine use suggests that this therapy may be suitable for those with such experimental recreational ketamine experiences. Ketamine use rates are high in the United Kingdom, where the study was conducted, with lifetime rates at 1.9% (
37). Because rates will likely be still higher in a group with AUD, particularly among 16- to 24-year-olds (
37), excluding individuals with any prior ketamine experience may become increasingly problematic if this is to be a widely adopted treatment.
Nearly half of our sample reported experimental use of psilocybin or LSD; individuals’ previous experiences with other psychedelic substances may have influenced expectations from ketamine treatment.
A formal assessment of the effect of therapeutic alliance would be a further important addition to future studies. The use of the Mystical Experience Questionnaire was not considered at the time we designed the study as this did not relate to our hypotheses, and we made the decision not to use the Clinician-Administered Dissociative States Scale in order to keep to a minimum the measures that participants were completing under the influence of ketamine. In retrospect, however, it would have been helpful to include these, and they would be an important addition to future studies. Given heterogeneity in baseline alcohol use, future studies might consider using individualized markers of drinking, such as total number of drinks consumed or multiple event approaches such as number of days of heavy drinking. Lastly, a dose-finding study might be an important avenue for future research, given the absence of such a study in AUD.
In summary, this trial demonstrated that three subanesthetic infusions of ketamine supported abstinence from alcohol and that abstinence may possibly be further enhanced when ketamine treatment is combined with therapy. Overall, the treatment was well tolerated. The data presented here, along with emerging data from other studies of ketamine in AUD, suggest that a further definitive trial is warranted.