Binge drinking (defined for men as drinking five or more drinks on one occasion), also known as heavy episodic drinking, accounts for more than half of the 80,000 annual deaths attributed to excessive alcohol consumption in the United States (
1). In 2018, the prevalence of past-30-day binge drinking was 17% among U.S. adults, and adults who binge drink reported, on average, 4.6 episodes of binge drinking per month (
2). Binge drinking is more common among some key HIV populations, including sexual and gender minority men (SGM)—including men who have sex with men (
3) and transgender men (
4)—who also account for over half of new HIV infections in the United States (
5). Binge drinking among SGM is independently associated with HIV-related sexual risk behaviors and HIV infection (
6–
8). Therefore, effective interventions to reduce binge drinking among SGM may also be important HIV prevention strategies (
9). Additionally, SGM may also have higher prevalences of other substance use compared with heterosexual adults (
10). This underscores the need to identify substances used by SGM that may be contraindicated with current pharmacologic treatment options, as well as the importance of identifying pharmacotherapies that may be helpful for multiple classes of substance use for SGM (
9).
Naltrexone is approved by the U.S. Food and Drug Administration (FDA) to treat alcohol use disorder (AUD), as well as opioid use disorder (
11). Its mechanism of action as an opioid antagonist involves the blockade of mu-opioid receptors from alcohol-associated endorphins, which in turn modulates the release of dopamine that would otherwise occur following alcohol intake, thereby attenuating some of the positive neurobiological effects of alcohol (
11). Reductions in the intensity of alcohol-related “high,” euphoria, and also craving are thought to contribute to naltrexone’s efficacy in the treatment of AUD (
11). Nevertheless, it remains underutilized as an intervention to reduce alcohol consumption; less than 10% of patients with AUD in the United States have ever received medications such as naltrexone (
12). Furthermore, of the 45 clinical trials of naltrexone for alcohol treatment, only a handful of studies have evaluated naltrexone for individuals who do not meet criteria for alcohol dependence or severe AUD (i.e., those with mild to moderate AUD) (
13–
16). This represents a notable imbalance in research, because the vast majority of individuals who binge drink (90%) do not meet criteria for severe AUD (
17).
Currently, the standard regimen for oral naltrexone is daily dosing, which creates barriers to adherence (
11,
18). Long-acting injectable naltrexone is an available alternative to oral dosing, although it requires a monthly injection administered by a clinician (
18). For patients who prefer oral naltrexone, alternative dosing schedules have been proposed, including targeted administration of naltrexone, whereby patients are instructed to take the medication as needed, in anticipation of heavy drinking episodes (also known as event-driven dosing) (
14). Targeted naltrexone administered after a period of daily dosing has been found to be significantly associated with reduced relapse to heavy drinking among nonabstinent individuals with severe AUD (
19). In another trial, men assigned to targeted naltrexone showed significant reductions in mean drinks per day and in number of drinks during drinking days, compared with those randomized to daily naltrexone administration, daily placebo, or targeted placebo (
14). A pilot study conducted by our research group (
20) found that taking targeted naltrexone on average at least three times weekly was significantly associated with a reduction in number of binge-drinking days compared with placebo. That study also demonstrated that it was feasible and acceptable to enroll SGM who currently binge drink and do not meet criteria for alcohol dependence in a pharmacotherapy clinical trial, with high retention and visit completion rates. Moreover, the study demonstrated that targeted naltrexone was significantly associated with reductions in HIV-associated sexual risk behaviors among SGM. Taken together, these studies supported a larger efficacy trial of targeted naltrexone in SGM.
Methods
Study Design and Recruitment
This was a randomized double-blind placebo-controlled trial with 1:1 random parallel-group assignment to targeted dosing of 50 mg of oral naltrexone or matching placebo. Participants were recruited via street outreach, recruitment flyers, sexual health clinics, needle exchanges, community organizations, bars, websites, and social media. Additionally, SGM participants who participated in an epidemiological study of alcohol use (
23) were invited to participate in this study. Potential participants completed a brief telephone screen to assess initial eligibility and, if eligible, were scheduled for an in-person screening visit. All participants gave informed consent using consent forms approved by the Institutional Review Board of the University of California, San Francisco. Based on data from our pilot study showing approximately constant differences between treatment and control groups over the course of the trial, and retention of 90% of participants at 12 weeks, we estimated that the target sample size of 120 participants would have 80% power in two-sided tests with a type I error rate of 5% to detect a 27% reduction in number of binge-drinking days, a 10% reduction in average numbers of drinks, and a 15%–23% reduction in alcohol urine positivity rate. We also estimated that the study would have 80% power to detect a 31% reduction in number of male anal sex partners, a 57% reduction in serodiscordant condomless anal intercourse partners, a 46% reduction in condomless anal intercourse partners while intoxicated with alcohol, and a 56% reduction in condomless anal intercourse events with serodiscordant partners.
Study Participants
We screened for eligibility using the following inclusion criteria: male gender; self-reported anal intercourse with men in the past 3 months while under the influence of alcohol; at least one binge-drinking episode (five or more drinks on a single occasion) per week in the past 3 months; interested in reducing binge alcohol consumption; less than three alcohol dependence symptoms in the Structured Clinical Interview for DSM-IV (SCID-IV); no current acute illnesses requiring prolonged medical care; no chronic illnesses likely to progress clinically during trial participation; able and willing to provide informed consent and adhere to visit schedule; age between 18 and 70 years; and a baseline complete blood count and comprehensive metabolic panel without clinically significant abnormalities as determined by the study clinician in conjunction with symptoms, physical examination, and medical history. Both HIV-negative and HIV-positive participants were eligible for the study.
Study exclusion criteria were as follows: any psychiatric condition (e.g., depression with suicidal ideation) or medical condition that would preclude safe participation in the study; known allergy to or previous adverse reaction to naltrexone; current use of or dependence on any opioids or a known medical condition that currently requires or may likely require opioid analgesics; opioid-positive urine screen at enrollment; current CD4 count <200 cells/mm3; moderate to severe liver disease (AST, ALT ≥3 times upper limit of normal); impaired renal function (creatinine clearance <50 mL/min); currently participating in another interventional research study with potential overlap; alcohol dependence as determined by SCID-IV criteria; not having a cell phone or not willing to use a study cell phone that could send and receive text messages; and any condition that, in the principal investigator’s and/or study clinician’s judgment, would interfere with safe study participation or adherence to study procedures.
Study Procedures
All study procedures were conducted at the San Francisco Department of Public Health. At screening visits, after completing informed consent, participants completed a medical history, measurement of vital signs, physical examination, comprehensive metabolic panel, and complete blood count. Participants who reported being HIV-negative or did not know their HIV status received HIV rapid testing (OraQuick HIV 1/2, OraSure Technologies, Inc., Bethlehem, Pa.) and pooled HIV RNA testing; HIV-positive participants received CD4 count testing. Participants received HIV risk reduction or “Prevention With Positives” counseling, as appropriate, based on Centers for Disease Control and Prevention guidelines (
24). Participants were evaluated for any psychiatric or medical condition that would preclude safe study participation, and other eligibility criteria. Eligible participants were scheduled for enrollment.
At enrollment, treatment was assigned using computer-generated, double-blinded block randomization using randomly selected block sizes of 2–4. The study statistician provided the randomization codes to the Safeway compounding pharmacy, which prepared kits corresponding with the treatment assignment (50 mg oral naltrexone or matching placebo) from the randomization code. The study clinicians, staff, and participants were all unaware of the treatment assignments for the study drug kits, and hence the study was double-blinded. Study clinicians provided training and instructions on targeted dosing of medication during enrollment. Participants were instructed to take one pill during periods of alcohol craving and/or when they perceived a high risk for heavy alcohol consumption (i.e., when binge drinking was anticipated), consistent with our pilot study (
20). Medications were dispensed in bottles with Medication Event Monitoring System (MEMS) caps (Aardex Group, Liège, Belgium), which are wireless medication monitoring devices that record each opening as a real-time medication event (
25). All participants were asked about potential adverse events at each follow-up visit, and symptom-driven physical examinations and safety laboratory monitoring were done at weeks 4, 8, and 12. Adverse events were classified using the Division of AIDS Table for Grading Severity of Adult Adverse Experiences for HIV Prevention Trials Network (
26). Behavioral assessments were conducted weekly and monthly, using audio computer-assisted self-interviews to standardize data collection and minimize reporting bias (
27).
Participants received medication management counseling for 12 weeks. Trained staff, supervised by a clinical psychologist, administered brief (20–30 minutes) alcohol use counseling at follow-up visits, which was modified from a standardized, manualized version of the medication management brief counseling platform used in the National Institute on Alcohol Abuse and Alcoholism’s COMBINE study (
28). In the COMBINE study, participants received nine sessions of medication management at baseline (week 0) and at weeks 1, 2, 4, 6, 8, 19, 12, and 16 (
28). For this study, we modified the frequency of medication management to be similar to our other pharmacotherapy trials, in which each weekly visit was paired with a counseling session (i.e., 12 sessions at weeks 0–11) (
29,
30). Medication management is a low-intensity supportive program designed to increase problem recognition and enhance motivation to change maladaptive alcohol use patterns. HIV risk-reduction counseling and testing were repeated for HIV-negative participants at the final visit.
Participants were paid $20 for each screening visit, $60 for enrollment, $20 for weekly visits, $45 for month 1 and 2 visits, and $60 for the final visit. The study was conducted under the FDA Investigational New Drug exemption (PIND no. 123842). Procedures were approved by Human Research Protection Program of the University of California, San Francisco (IRB no. 14-14481), and the trial was registered at ClinicalTrials.gov (NCT02330419).
Outcome Measures
For feasibility outcome measures, we computed the proportions of participants eligible and enrolled among those recruited and screened, the proportion of scheduled visits completed, and the proportion of participants retained to the end of the study. We also tabulated the proportion of participants in each group who correctly guessed their treatment assignment to determine whether there was significant evidence of unblinding at the end of the trial.
Acceptability measures were computed from questions on attitudes about trial participation and level of satisfaction with trial procedures. We recognize that measuring adherence objectively is difficult for studies with as-needed dosing. Consistent with our pilot study, we collected data on self-reported as-needed dosing by having participants report the number of days when they used their study medication and thought that they had high risk for heavy drinking and/or craved alcohol (
20). To objectively track the frequency of taking the study drug, we also measured the number of MEMS cap openings during treatment. Cumulative percent adherence was calculated by dividing the frequency of openings at a given time point by the number of days since enrollment. For tolerability measures, we computed the proportion of those who experienced adverse events, both overall and by type.
Self-reported alcohol measures from each day within the preceding week and month were measured using a modified timeline followback method (
31). To assess patterns of alcohol use outcomes, we used the following measures: number of drinks in the past 30 days, number of drinking days in the past week, number of binge-drinking days in the past week, and any binge drinking in the past week. We used two biomarkers, ethyl glucuronide (EtG) and phosphatidylethanol (PEth), as objective measures for recent and long-term abstinence from alcohol, respectively. We examined the proportion of alcohol metabolite–positive urine screens each week by testing for EtG positivity, using the cutoff of 100 ng/mL (
32). EtG testing was conducted by Redwood Toxicology Laboratories (Santa Rosa, Calif.). Urine EtG testing is estimated to have the ability to detect alcohol use from the past 1 to 3 days (
32). Additionally, we examined alcohol consumption by testing for PEth concentrations in dried blood spot samples; PEth levels were dichotomized as negative or positive for alcohol use using the cutoff of 20 ng/mL (
32). PEth, a phospholipid formed only in the presence of alcohol, is a novel, direct biochemical marker of alcohol that has shown high sensitivity and specificity in detecting alcohol use over a period of 2–3 weeks. We collected dried blood spot samples at baseline and weeks 3, 6, 9, and 12 and sent the samples to United States Drug Testing Laboratories (Des Plaines, Ill.) for PEth testing. The alcohol outcomes preregistered at ClinicalTrials.gov were number of binge-drinking days and EtG-positive urine samples.
The current recommendations on statistical principles in clinical trials indicate that it is appropriate to select primary endpoints that are “capable of providing the most clinically relevant and convincing evidence directly related to the primary objective of the trial” (
33,
34). As noted, the primary object of this trial was to examine the efficacy of oral naltrexone in reducing binge drinking. Therefore, the primary endpoints that correspond with this primary objective are the following outcomes that measure binge-drinking intensity: 1) number of drinks in the past 30 days; 2) any binge drinking in the past week; 3) number of binge-drinking days in the past week; and 4) number of drinking days in the past week. This is also consistent with the FDA guidelines for clinical trials with multiple endpoints, which differentiate between endpoints that are considered primary outcomes, based on primary study objectives, and secondary outcomes, which may include endpoints that support the mechanism of the intervention or describe processes related to the primary outcomes (
35).
Standardized measures were used to assess other secondary outcomes, including alcohol craving via the alcohol craving visual analogue scale (a validated measure for subjective alcohol craving or desire to drink in the past 24 hours [
36], which has been shown to be associated with increased alcohol use when elevated [
37]), hazardous alcohol use via the Alcohol Use Disorders Identification Test (AUDIT-10), severity of dependence via the Severity of Alcohol Dependence Scale, and sexual risk behavior using published measures from our prior studies (
31,
38–
40). For monthly visits, participants completed the measures related to depression using the Center for Epidemiologic Studies Depression Scale (CES-D) (
41). Participants were also seen at posttreatment visits 6 months after their week 12 visit to examine durability of treatment effects. Measures and specimens similar to those described above (e.g., alcohol outcome measures, behavioral measures; EtG urine and PEth dried blood spot samples) were collected at posttreatment visits.
Statistical Analysis
We used Wilcoxon rank sum and Fisher’s exact tests, as appropriate, to assess the comparability of participants by treatment assignment at baseline, as well as differences in feasibility, acceptability, and tolerability measures. We compared cumulative adherence between treatment groups using the Wilcoxon test.
In intention-to-treat analyses, we conducted a priori exploratory assessments using generalized estimating equation (GEE) Poisson models with robust standard errors to account for within-subject correlation, to assess treatment effects on alcohol use outcomes ascertained using timeline followback data and results from alcohol urine metabolite and dried blood spot analyses. The primary intention-to-treat analyses excluded outcomes from week 1. In all models, the average effect of the intervention was estimated by the interaction between treatment assignment and an indicator variable for follow-up visit. These models provided incidence rate ratios of our outcomes of interest. We also calculated number needed to treat by calculating the inverse of the absolute rate differences from the models described above on outcomes that were statistically significant (
42). For the outcome number of drinks in the past 30 days, we rescaled this number-needed-to-treat measure to 10 drinks to facilitate interpretability.
In post hoc analyses, to account for multiple hypothesis testing, we used the false discovery rate (FDR) using the Benjamini-Hochberg procedure, which controls for the expected number of type I errors (i.e., false positive findings) (
43,
44). Consistent with FDA guidelines for clinical trials with multiple endpoints (
35), and as recommended by Zhang and colleagues (
45), multiple-testing p-value corrections were applied for the hypotheses that tested the primary outcome endpoints (i.e., outcomes related to binge-drinking reduction).
We also fitted models on these outcomes at 6 months posttreatment to evaluate evidence of sustained treatment effects. In sensitivity analyses, we repeated these analyses adjusting for baseline correlates of missing data, and we imputed missing EtG and PEth outcomes as positive.
In addition, we conducted as-treated analyses examining the treatment effects of naltrexone compared with placebo among participants whose number of MEMS openings was above the median, which corresponded to an average of at least 2.5 openings per week.
We also evaluated treatment effects on alcohol-associated sexual risk behaviors using GEE Poisson, binomial, and negative binomial models using intention-to-treat analyses. We used GEE models to explore whether naltrexone was associated with reduction in depressive symptom scores and hazardous alcohol consumption, as measured by the CES-D and the AUDIT-10, respectively. We also assessed whether naltrexone was associated with reduced alcohol craving and severity of alcohol dependence, using similar methods. Analyses were conducted in STATA, version 15.0 (StataCorp, College Station, Tex.).
Results
Screening and Enrollment
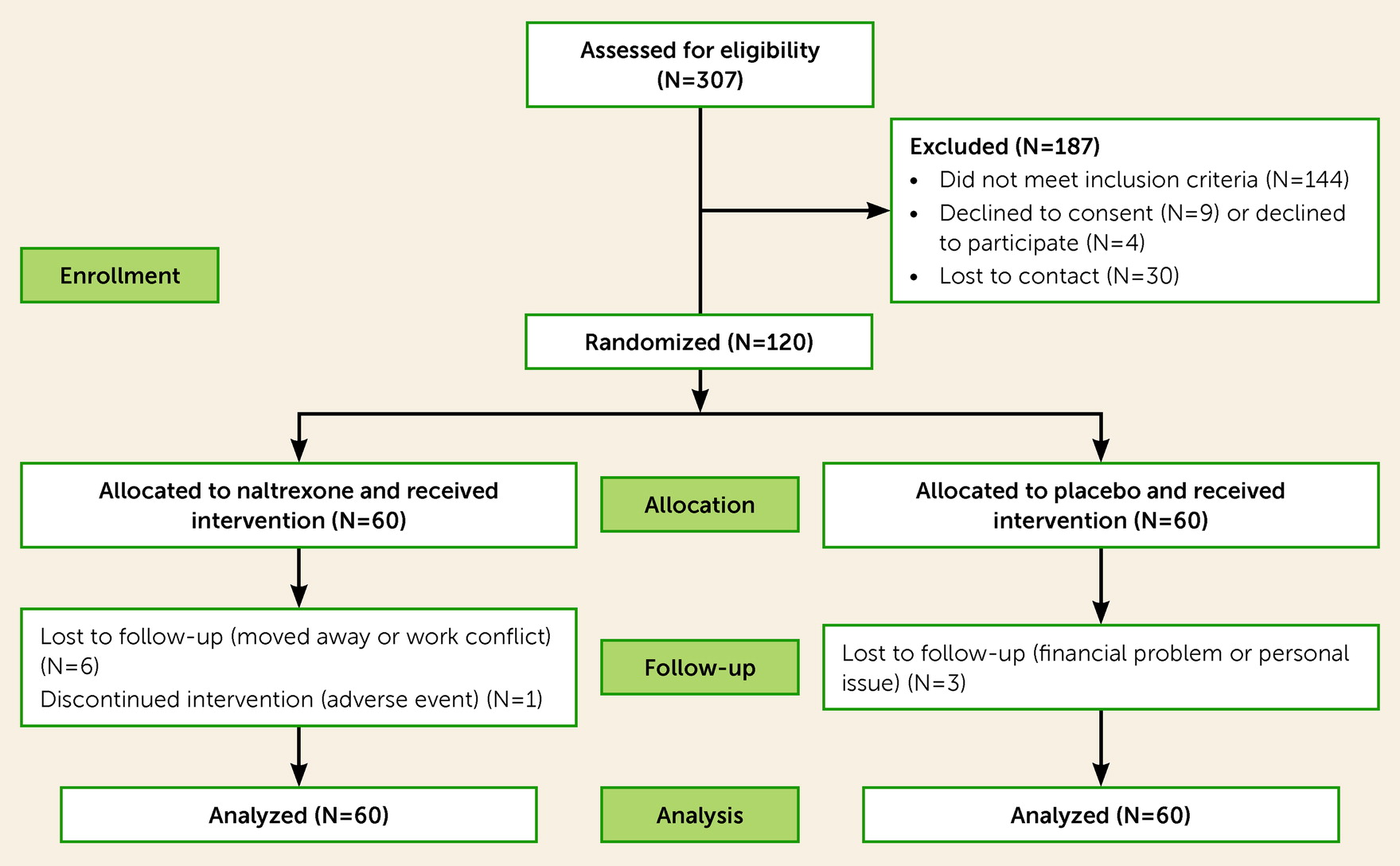
Figure 1 shows results for screening, recruitment, assignment, and retention for the study period from May 2015 to November 2020. A total of 307 people were assessed for eligibility and consented to participate, of whom 144 were deemed ineligible because they did not meet one or more inclusion criteria (some participants may be deemed ineligible for more than one reason). The most common reasons for ineligibility were current alcohol dependence (N=106); participation in another ongoing study (N=14); clinical judgment deeming that participation would be unsafe (N=8); binge drinking less than four times per week (N=6); and not having anal intercourse under the influence of alcohol (N=5). Less common reasons for ineligibility included having a safety laboratory test (e.g., via complete blood count or metabolic panel results) meeting exclusion criteria (N=4), residing outside the San Francisco Bay Area (N=2), major depression with suicidal ideation (N=2), not interested in stopping or reducing drinking (N=1), having a psychotic disorder (N=1), having another psychiatric condition (N=1), and being newly diagnosed with HIV during study screening (N=1). In addition, 30 people were lost to follow-up during screening and 13 declined participation. In total, 120 participants (39% of those screened) were randomized (60 to naltrexone, 60 to placebo).
Participant Characteristics
The trial recruited a diverse sample of SGM (54% White, 10% Hispanic/Latino, 14% Black, 5% Asian and Pacific Islander, and 17% mixed or another race), of whom 26% were HIV positive (
Table 1). The participants’ median age was 37 years (IQR=30–45). The trial enrolled one transgender male and 119 cisgender participants. Baseline demographic characteristics were similarly distributed in both groups (p>0.05 for all). Baseline urine screens found that 22% (N=27) of participants tested positive for marijuana, 14% (N=17) for cocaine, 8% (N=10) for methamphetamine, 6% (N=7) for amphetamine, and 6% (N=7) for benzodiazepine metabolites. None of the samples screened positive for opioids. There were no significant differences in urine positivity across these substances between groups (p>0.05 for all; data not shown).
Retention
Overall, the percentage of weekly study follow-up visits completed was 85% (1,218 of 1,440 weekly visits). Percentages of weekly follow-up visits completed were similar between the naltrexone (84%; 606 of 720) and control groups (85%; 612 of 720) (p=0.72). Overall, 111 participants (93%) were retained at the end of the study, and the study completion rate was similar between the two groups (naltrexone group: 90% [54/60]; placebo group: 95% [57/60]; p=0.49). Six-month posttreatment visit retention was 84% overall, and retention rates were similar between groups (naltrexone group: 83% [50/60]; placebo group: 85% [51/60]; p≥0.99).
Acceptability of Procedures
Overall, 84% of participants reported being satisfied or highly satisfied with their study participation. In addition, 82% reported that they would be interested in participating in a similar study in the future, and 91% reported that they would likely recommend participation in the study to a friend. Acceptability of study procedures was similar between the treatment groups.
Adherence
On average, participants reported taking study medication 73.65% (SD=34.75) of the days that they craved alcohol or anticipated a heavy drinking session; results were similar between the treatment groups (naltrexone group: 71.05%, SD=37.56; placebo group: 76.19%, SD=31.59; p=0.17). Additionally, the mean number of study medication doses taken by participants as measured by MEMS cap data was 31.23 (SD=18.38) and was similar between groups (naltrexone group: 31.85, SD=20.60; placebo group: 30.62, SD=16.06; p=0.305).
Assessment of Unblinding
In the naltrexone group, 39% guessed that they were on naltrexone, and in the placebo group, 59% guessed that they were on placebo; however, the difference between groups was not statistically significant (p=0.054).
Tolerability
The overall frequency of adverse events was similar between the two groups (p=0.08). There were two serious adverse events in the study, both deemed unrelated to naltrexone. One serious adverse event was in a participant in the placebo group who was hospitalized for bowel obstruction (grade 4 adverse event) at month 2 of treatment. The other serious adverse event occurred in a participant in the naltrexone group who developed a soft-tissue infection that resolved after a course of antibiotics. The most frequently observed adverse events in both groups were nausea (naltrexone group, N=14; placebo group, N=4; p=0.02), hyperglycemia (naltrexone group, N=8; placebo group, N=9; p>0.99), headaches (naltrexone group, N=7; placebo group, N=2; p=0.16), increased ALT (naltrexone group, N=5; placebo group, N=3; p=0.72), and increased AST (naltrexone group, N=6; placebo group, N=2; p=0.27). Nausea and headaches are both known side effects of naltrexone. The most frequently observed adverse events that were present only in the naltrexone group were rash (N=3) and diarrhea (N=3), although the difference between treatment groups was not statistically significant (p=0.53 for both adverse events).
Intention-to-Treat Efficacy Analyses of Alcohol Use Outcomes
Binge-drinking outcomes.
In intention-to-treat analyses (
Table 2), targeted naltrexone significantly reduced the reported number of binge-drinking days compared with placebo during the 12-week follow-up (incidence rate ratio [IRR]=0.74; 95% CI=0.56, 0.98; p=0.03; number needed to treat [NNT]=2 to prevent a binge drinking day each week). Moreover, the naltrexone group on average had significantly fewer weeks with any binge drinking (IRR=0.83, 95% CI=0.72, 0.96; p=0.01; NNT=7.4 to prevent a week without binge drinking). In addition, the naltrexone group had significantly fewer reported drinks per month (IRR=0.69, 95% CI=0.52, 0.91; p=0.01; NNT=5.7 to prevent 10 additional drinks per month). The number of drinking days in the past week was similar between the two groups. In post hoc analyses applying FDR correction for multiple hypothesis testing, naltrexone was significantly associated with reductions in number of binge-drinking days (FDR-adjusted p=0.045), fewer weeks with any binge drinking (FDR-adjusted p=0.02), and fewer reported drinks per month (FDR-adjusted p=0.036). Similar findings were observed in sensitivity analyses adjusting for baseline correlates of missingness.
Alcohol use biomarker outcomes.
The proportions of weekly EtG-positive urine samples and PEth-positive dried blood spots were similar between the two treatment groups. PEth concentrations were also similar between the two groups. Similar findings were observed in sensitivity analyses adjusting for baseline correlates of missingness.
Posttreatment outcomes.
In posttreatment analyses, we observed sustained treatment effects at the 6-month posttreatment visit in favor of naltrexone over placebo on number of drinks in the past 30 days (IRR=0.69, 95% CI=0.50, 0.97; p=0.03), number of binge-drinking days in the past week (IRR=0.67, 95% CI=0.47, 0.95; p=0.03), and any binge drinking in the past week (IRR=0.79, 95% CI=0.63, 0.99; p=0.04).
As-Treated Analyses of Alcohol Use Outcomes
In as-treated analyses, the efficacy of naltrexone in reducing any binge drinking in the past week (IRR=0.84, 95% CI=0.71, 0.99; p=0.04) and number of binge-drinking days in the past week (IRR=0.67, 95% CI=0.47, 0.96; p=0.03) remained statistically significant in favor of naltrexone (see
Table 2). In addition, in as-treated analyses, PEth concentrations were on average 55.47 ng/mL lower (95% CI=−110.75, −0.20; p=0.04) in the naltrexone group compared with the placebo group. As-treated analyses did not reveal significant treatment effects for number of drinks in the past 30 days, number of drinking days in the past week, or EtG positivity.
Effects on Alcohol Craving, Severity of Alcohol Dependence, Depression, and Sexual Behaviors
In intention-to-treat analyses, naltrexone was significantly associated with reductions in alcohol craving. Participants in the naltrexone group reported an average reduction of 9.25 points (95% CI=−17.20, −1.31; p=0.02) on the alcohol craving visual analogue scale during follow-up compared with those in the placebo group. Changes in AUDIT-10 score, Severity of Alcohol Dependence Scale score, CES-D score, and sexual behaviors were similar between the naltrexone and placebo groups.
Discussion
In this trial in SGM who binge drink and have mild to moderate AUD, targeted oral naltrexone was associated with significant reductions in number of binge-drinking days, frequency of weekly binge-drinking episodes, number of alcoholic drinks consumed, and alcohol craving intensity. Additionally, this study demonstrated acceptability and feasibility of targeted use of naltrexone as a chemoprophylaxis strategy to manage periods of alcohol craving or at events when heavy drinking is anticipated. Moreover, the study showed sustained reductions in alcohol use patterns 6 months after treatment, suggesting that targeted naltrexone can result in lasting benefits for this population. Hence, this study supports the use of a targeted dosing approach for naltrexone in SGM who are interested in reducing their heavy alcohol consumption on an event-driven, as-needed basis.
These results are broadly consistent with previous studies in general adult populations with AUD, which have reported reductions in heavy alcohol use and craving associated with naltrexone treatment (
11,
46). The present study builds on those trials by demonstrating the utility of naltrexone in addressing binge drinking and event-level alcohol use patterns among SGM with mild to moderate AUD, and by demonstrating the efficacy of a targeted dosing approach for this population. The efficacy associated with targeted dosing of naltrexone is also consistent with studies on another opioid antagonist, nalmefene, which have demonstrated the efficacy of as-needed pharmacotherapy in reducing heavy episodic drinking in the general population (
47,
48). The posttreatment findings of the present study differ somewhat from those of studies that include participants with severe AUD, in which sustained treatment effects from naltrexone were not observed (
49). We speculate that posttreatment reductions in alcohol use may be more difficult to maintain long-term among individuals with severe AUD, compared to our study population with mild to moderate AUD, which may explain the differences between these findings.
Our study also found that targeted naltrexone dosing was tolerable and safe for SGM who are currently binge drinking with mild to moderate AUD. We did not observe any serious adverse events due to naltrexone, and overall the frequencies of adverse events were similar between the naltrexone and placebo groups (with the exception of nausea, which is a known side effect of naltrexone). While nausea was more common in the naltrexone arm, all nausea events were mild or moderate and resolved as participants adjusted to naltrexone treatment. These findings are consistent with other clinical trials of naltrexone (
50). Notably, we observed favorable uptake of targeted naltrexone. Based on MEMs cap data, study medication was taken about 2.5 times weekly, and by self-report, participants took the medication prior to more than 70% of events when they anticipated a risk of heavy alcohol use, indicating a high level of medication coverage for event-driven dosing. These findings are consistent with our pilot study on targeted naltrexone, which documented similar acceptability outcomes for as-needed medication use among individuals with mild to moderate AUD.
We did not observe significant treatment effects in weekly EtG urine and PEth dried blood spot positivity. However, among participants who took the study medication at least 2.5 times weekly, PEth concentrations were on average lower by 55.47 ng/mL in the naltrexone group compared with the placebo group. These findings suggest that while there were no differences in alcohol abstinence between groups, those who took naltrexone more frequently likely had reductions in alcohol consumption. These findings are broadly consistent with a meta-analysis of 64 studies that found that naltrexone was efficacious in reducing alcohol consumption, but it had a lower effect size for alcohol abstinence relative to another pharmacotherapy (acamprosate) (
46). Importantly, we did not require that participants abstain or express interest in abstaining from alcohol to participate in our trial. As noted in the meta-analysis cited above, requiring alcohol abstinence before the trial was associated with larger effect sizes for abstinence maintenance in naltrexone trials (
46). Studies on targeted naltrexone in individuals interested in achieving alcohol abstinence may be needed to determine whether the findings of that meta-analysis also translate to targeted naltrexone. Nevertheless, despite the lack of treatment effects on alcohol abstinence, the reductions in alcohol consumption associated with naltrexone are important given the accumulating evidence that reductions in drinking are also associated with improved clinical outcomes and quality of life, as well as reductions in alcohol-related problems (
51–
53).
Additionally, our study did not detect significant differences in sexual risk behavior between groups, despite the significant reductions in alcohol use. These results differ from our pilot study, in which we observed that naltrexone was associated with significant reductions in some sexual risk behaviors, such as receptive condomless anal intercourse. This unexpected finding may be partially explained by the lower overall number of sexual partners among participants compared with our previous studies. This may have lowered the present study’s statistical power to detect differences between arms, and our sample size was only powered to detect medium to large effect sizes on sexual behavior outcomes. Additionally, it is possible that the threshold of alcohol reduction needed to influence changes in sexual risk behaviors may be greater than the reductions that occurred in our study. For instance, a meta-analysis has estimated that on average, a 0.1 mg/mL decrease in blood alcohol concentration is associated with a 5% decrease in reported likelihood of engaging in condomless sex (
54). Although we did not measure blood alcohol concentration in this study, it is likely that greater reductions in drinking may be necessary to correspond to the decline in blood alcohol concentration associated with significant effects on sexual behaviors.
Our study has several important limitations. Our study was conducted in a sample comprising SGM in the San Francisco Bay Area, which may limit the generalizability of our findings among other SGM or other individuals who binge drink and have mild to moderate AUD. Studies outside the San Francisco Bay Area and inclusive of other populations with mild to moderate AUD may be needed to examine the potential of targeted naltrexone more broadly. Additionally, our self-reported alcohol measures may be subject to bias. However, these measures have been validated, and we used audio computer-assisted self-interviews to further enhance the validity of our self-reported measures. There is also evidence that collecting objective alcohol biomarkers can enhance the validity of self-report of alcohol use and reduce social desirability bias (
55). Another limitation stems from the window of detection for alcohol positivity from EtG, which is limited to 1–3 days, which may have resulted in our study missing weeks when drinking occurred. However, because we also tested for PEth concentrations, which had a 3-week window for detection of alcohol use, we are more confident in our ability detect differences in alcohol abstinence in our study.
Additionally, the study did not examine the efficacy of targeted naltrexone compared with daily naltrexone among SGM. This head-to-head comparison may be an important future research direction that could be informative on the best dosing approaches for SGM who binge drink and have mild to moderate AUD. Finally, although we conducted post hoc FDR adjustments for outcomes relevant to the main goals of the study (i.e., binge-drinking outcomes), we acknowledge that our study did not include correction rules for multiple comparisons a priori. Hence, our findings should be interpreted with caution. However, the consistency of our findings with other studies on oral naltrexone as well as the parallel reductions between craving, which has been shown to predict drinking (
36), and alcohol use patterns provide us with additional confidence in the validity of these results.
Despite these limitations, this study builds on the literature by demonstrating the efficacy of targeted oral naltrexone in reducing alcohol use and craving among SGM who currently binge drink and meet criteria for mild to moderate AUD. The findings from our study have several implications in clinical practice. First, they support the use of oral naltrexone as a treatment approach for the population who comprise the majority of binge drinkers (i.e., those who do not meet criteria for severe AUD). Expanding naltrexone treatment access to these individuals can help address the public health consequences associated with binge drinking, especially among communities with high binge-drinking prevalence rates, such as SGM. The feasibility, acceptability, and efficacy findings of this study on the targeted dosing approach support alternative (i.e., nondaily) dosing regimens for current alcohol use disorder pharmacotherapies, which may in turn help expand uptake of pharmacotherapy by providing an additional option for individuals who may be interested in event-driven dosing. Indeed, this approach has been used to expand the use of other chemoprophylaxis treatments, including, for example, medications for HIV prevention (
56).
Additionally, targeted dosing of naltrexone can also be considered as one option alongside different formulations of naltrexone, depending on the patient’s drinking patterns and preferences for dosing. For example, long-acting-injectable dosing may be attractive to those who are open to monthly injection visits to their clinicians and those who may engage in heavy drinking more frequently, while targeted dosing may be appealing to those who are not open to monthly injection visits or who engage in more heavy episodic drinking patterns that may be amenable to event-driven, targeted dosing (
11,
18). Other medications may also be more suitable, depending on the patient’s individual goals for drinking (for example, acamprosate may be preferred over naltrexone for patients who are interested in abstaining from alcohol) (
46). Moreover, naltrexone may be well-suited for individuals with AUD and opioid use disorder, but only if they are able to abstain from opioids (
11,
18); otherwise, patients with opioid use disorder may respond better with acamprosate, which does not require opioid abstinence (
46). Future studies exploring preferences (e.g., discrete choice experiments that examine preferred treatment attributes [
57]) for pharmacotherapy dosing can also help inform development of optimal approaches for alcohol treatment.
In summary, we found that targeted use of oral naltrexone was efficacious in reducing alcohol use and craving among SGM who binge drink and have mild to moderate AUD, with sustained effects 6 months after treatment. Retention and treatment engagement were also high. Taken together, these data support the use of targeted dosing of oral naltrexone to address binge drinking in SGM with mild to moderate AUD. Efforts to expand access to this treatment approach can enhance public health efforts to address binge drinking and associated harms.