Hallucinogenic drugs have been reportedly used by indigenous peoples for millennia for spiritual purposes, shamanism, and healing (
1–
3). In the 1950s and 1960s, psychiatry utilized hallucinogenic drugs as tools to interrogate various neurotransmitter systems and their roles in neuropsychiatric diseases (
4–
6). Thus, for example, early studies on psychedelic drugs postulated that drugs such as
N,N-dimethyltryptamine (DMT) (
7) and lysergic acid diethylamide (LSD) (
6,
8) might induce a model psychosis in humans and laboratory animals. More recently, psilocybin—the active ingredient of “magic mushrooms” (
Psilocybe spp.)—has been shown in several phase 2 clinical trials to robustly and rapidly alleviate depressive symptoms (
9–
13). Initial clinical studies with LSD have shown a similar rapid action for symptoms of anxiety (
14) in terminal cancer patients. In this review, we summarize our current understanding of psychedelic drug actions and how such insights might inform further research and the potential clinical utility of psychedelic drugs.
What Is a Psychedelic Drug?
The term psychedelic was coined by Osmond in 1957 (
15) to refer to drugs that are “mind manifesting.” Prior to this, psychoactive drugs such as hallucinogens and dissociative agents (among others) were considered to be psychotomimetic (
16), a term that is in common use today. Osmond defined psychotomimetic compounds as follows:
Psychotomimetic agents are substances that produce changes in thought, perception, mood and, sometimes, in posture, occurring alone or in concert, without causing either major disturbances of the autonomic nervous system or addictive craving, and although, with overdosage, disorientation, memory disturbance, stupor, and even narcosis may occur, these reactions are not characteristic (
15, p. 418).
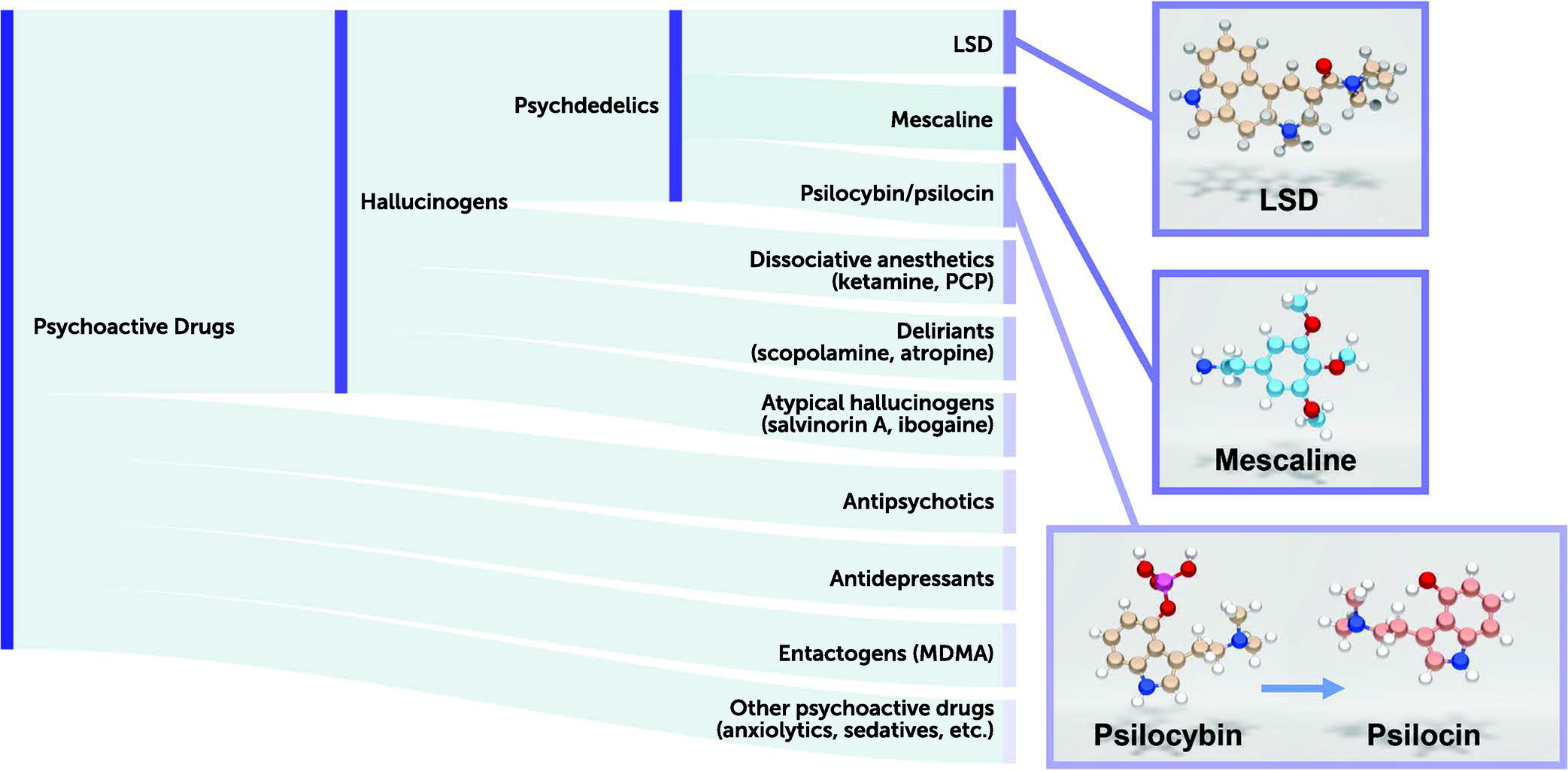
Of the various so-called psychotomimetic drugs, Osmond included such compounds as the classic psychedelics (e.g., LSD, psilocybin) as well as the hallucinogens ibogaine (from the iboga plant), mescaline (from the cactus
Lophophora williamsii), DMT (from several plant species, and used in hallucinogenic snuff), and atropine (from the mushroom
Amanita muscaria) (
15). To this list are added synthetic compounds such as the dissociative anesthetic agent ketamine (
17), the hallucinogenic kappa opioid receptor agonist salvinorin A (from the plant
Salvia divinorum) (
2), and others (
Figure 1).
Our current classification of these psychoactive drugs would include hallucinogens as a distinct class (
Figure 1), with many different types of hallucinogens based primarily on their pharmacology. Thus, psychedelic drugs are defined as drugs that have an LSD-like action in humans and are 5-HT
2A agonists (
3,
18). This definition is similar to that offered by the U.S. Food and Drug Administration: “serotonergic 5-HT
2A agonists that alter perception, cognition, and mood (i.e., psychedelic effects) and that are currently controlled in Schedule I of the Controlled Substances Act” (
19). The European regulatory authorities have provided a similar definition for classic psychedelic drugs as 5-HT
2A agonists, exemplified by drugs such as LSD, mescaline, and psilocybin (
20). By contrast, drugs such as ketamine and phencyclidine—which can also induce hallucinations along with dissociative states—are classified as dissociative anesthetic agents and are NMDA receptor antagonists. Muscarinic antagonists, such as scopolamine and atropine—which induce delirium and hallucinations in humans—are classified as deliriants. Finally, atypical hallucinogens that have kappa opioid receptor agonist activity (e.g., salvinorin A [
2]) constitute another class. MDMA (3,4-methylenedioxymethamphetamine; “Ecstasy”) is not considered a psychedelic drug (as it does not induce an LSD-like effect in humans) and has been classified separately as an entactogen (
21).
Psychedelic Drugs Mediate Their Actions via Serotonin 5-HT2A Receptors
There is now considerable evidence that a subclass of serotonin receptors, the 5-HT
2A subtype, is essential for the hallucinogenic actions of psychedelics. Initial evidence came from animal studies that indicated that 5-HT
2A antagonists block the actions of psychedelics (
22) and that their in vivo effects are directly correlated with their affinities for 5-HT
2A receptors (
23). Subsequent studies in which the 5-HT
2A receptor was genetically deleted showed that the effects of psychedelic drugs were blocked (
24,
25). The most definitive evidence comes from human studies in which the psychedelic actions of both psilocybin (
26) and LSD (
27) were blocked by pretreatment with the 5-HT
2A-preferring antagonist ketanserin.
Serotonin 5-HT
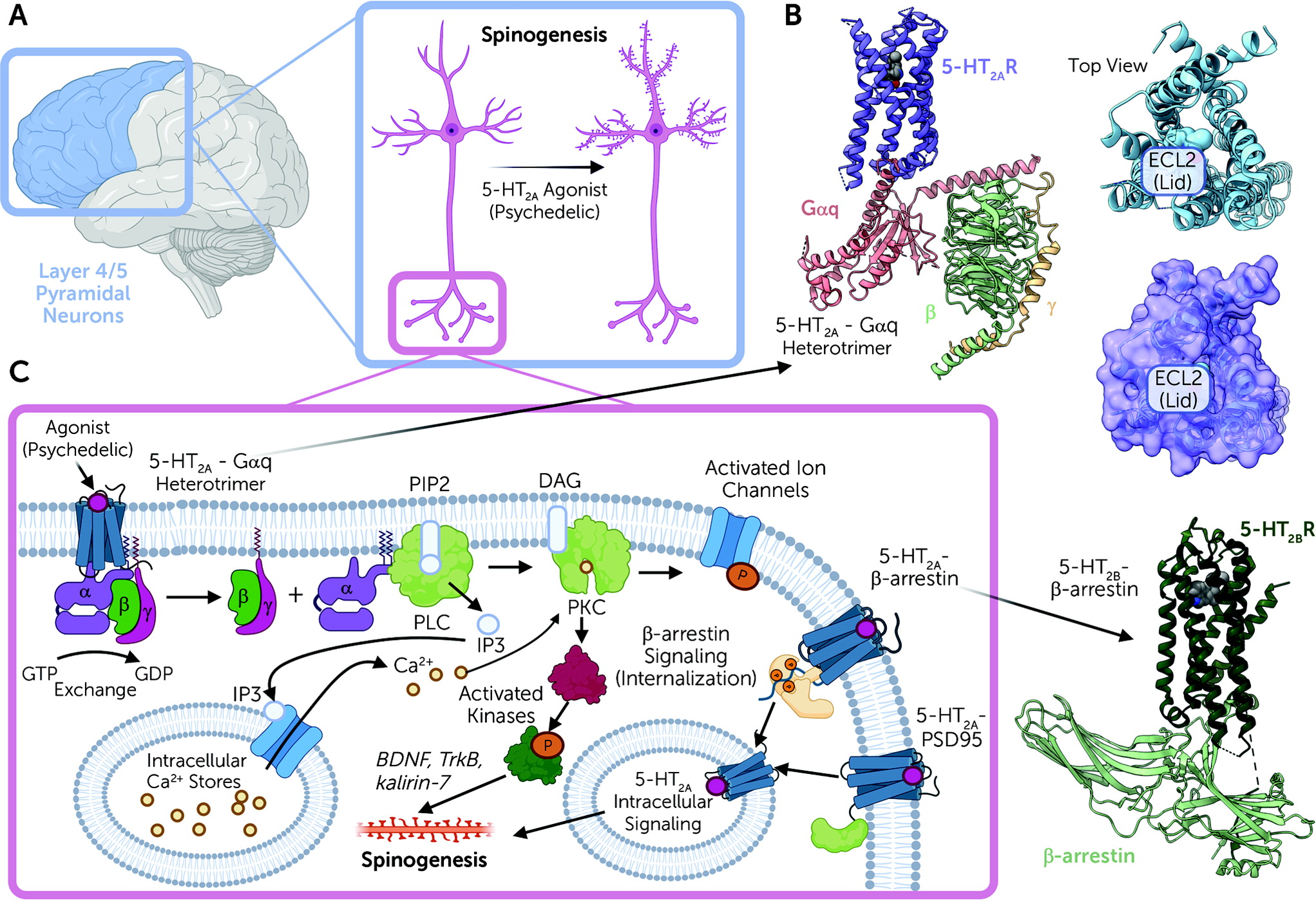
2A receptors are expressed mainly in layer 4 and 5 cortical pyramidal neurons, with sparse expression in parvalbumin-expressing interneurons (
28,
29) (
Figure 2A). In pyramidal neurons, 5-HT
2A receptors are concentrated in apical dendrites (
28,
29), complexed with various scaffolding proteins, including PSD-95 and others (
30). These interactions with scaffolding proteins are essential for the acute effects of psychedelic drugs in vivo (
30,
31). Intriguingly, many atypical antipsychotic drugs are potent 5-HT
2A antagonists (
32), and these same scaffolding proteins are also essential for the actions of clozapine-like atypical antipsychotic drugs in vivo (
30,
31).
In terms of the potential therapeutic actions of psychedelic drugs, the data are mixed regarding the necessity of 5-HT
2A receptor activation. One study in mice showed that ketanserin does not block the potential therapeutic actions of psilocybin (
33), while another showed complete blockade with ketanserin (
34). The latter study also showed lack of antidepressant-like actions in mice in which 5-HT
2A receptors are blocked. Such studies using ketanserin are problematic, however, as this agent also interacts with alpha-1 adrenergic receptors (
35) and with vesicular monoamine transporters sensitive to reserpine (
36). In future studies, the use of more selective 5-HT
2A antagonists—for example, pimavanserin, which is approved for treating Parkinson’s psychosis (
37,
38) and is the most selective approved 5-HT
2A antagonist (
37)—could be used to definitively address this question.
We now have molecular-level details regarding how psychedelic drugs interact with and activate 5-HT
2A receptors (
39) (
Figure 2B). Studies on a related serotonin receptor (5-HT
2B) have clarified how LSD can stabilize distinct signaling complexes (
40,
41). A key finding of these studies was the discovery that once LSD binds to the 5-HT
2A receptor, a lid is formed over the binding pocket, which “traps” LSD for several hours (
39,
40) (
Figure 2B). These findings imply that at least part of the reason for the long duration of action of drugs like LSD is the trapping of the receptor via conformational changes that occur after drug binding. These studies also showed that this prolonged action of LSD is due in part to a specific residue within the binding pocket, which is found in humans but not in mice or rats (
39). This residue (Ser242) also is essential for the high-affinity interactions of LSD, psilocybin, and perhaps other such drugs at the human and nonhuman primate 5-HT
2A receptors.
After psychedelic drug binding, 5-HT
2A receptors then activate a complex web of signaling processes mediated by interactions of the 5-HT
2A receptor with various transducer molecules, including both G proteins and arrestins (
42) (
Figure 2C). The main G protein activated is Gαq, which leads to the activation of phospholipase C (
43,
44) and mobilization of intracellular calcium, leading ultimately to enhanced neurotransmission at cortical pyramidal neurons (
45). In addition to G protein activation, 5-HT
2A receptors also induce arrestin interactions (
39,
41,
46). Interactions with both G proteins and arrestins appear to be essential for the full expression of psychedelic drug–induced behaviors in mice (
47,
48). Finally, there is evidence that psychedelic drugs may also induce changes in brain gene transcription (
49,
50), although it is unclear whether these effects are central to their putative therapeutic actions.
Psychedelic drugs also rapidly induce enduring changes in spine formation and dendritic arborization in layer 5 cortical pyramidal neurons (
51–
53). These findings are especially intriguing given the observations that 5-HT
2A receptors are enriched in layer 5 cortical neuronal synapses and dendritic arborizations at the microscopic (
54) and ultrastructural levels (
55). The molecular details regarding the mechanisms by which 5-HT
2A receptor activation induces enhanced plasticity are unclear, although pathways involving BDNF (
56), TrkB (
52), and kalirin-7 signaling (
51) (
Figure 2B) have been implicated. These findings take on added significance given the long-standing findings that antidepressant drugs enhance spine formation (
57–
59). Additionally, since antidepressant drug–induced spine formation appears to be central to the therapeutic actions of antidepressants (
60,
61), these same pathways could be involved in the antidepressant drug–like actions of psychedelics.
In a recent study by the Olson lab (
62) examining the role of psychedelics in inducing spine formation, the authors provide data consistent with the hypothesis that intracellular 5-HT
2A receptors are essential for the plasticity-inducing actions of psychedelics (
62). These findings are intriguing, as previous studies have shown that 5-HT
2A receptors in the brain are found in the dendroplasmic reticulum, where they interact with MAP1A (
55). Many anatomical studies have demonstrated a close association between intracellular 5-HT
2A receptors and various transducers and effectors, including arrestins (
63), RSK2 (
64), and many others (
65). Further research on the role of intracellular 5-HT
2A receptors in mediating the actions of psychedelics is warranted.
Problematic Off-Target Actions of Psychedelics
In addition to their actions at 5-HT
2A receptors, most psychedelic drugs have complex polypharmacological interactions with many other receptors in the brain (
25,
66,
67). Thus, for instance, LSD is a high-affinity agonist for nearly every serotonin, dopamine, and noradrenergic receptor (
67). In fact, LSD has been found to be a high-potency dopamine receptor agonist (
8,
68), with significant activity at both D
1 and D
2 family receptors (
67). DMT has a similarly robust agonist profile at several 5-HT receptors (
25), and it has been reported that its interactions with sigma-1 receptors may be involved in at least some of its actions in vivo (
69), although not its psychedelic effects (
25). Finally, psilocin (the active metabolite of psilocybin) has also been found to be a high-affinity agonist for most 5-HT receptors, including 5-HT
2C and 5-HT
2B (
70). In fact, many psychedelic drugs, as well as MDMA, activate 5-HT
2C receptors (
71–
76). Given that many 5-HT
2C agonists are anorectic (
77), the appetite-suppressant actions of some psychedelics could be related to this effect. What effects, if any, these off-target actions of psychedelic drugs have for their therapeutic actions is unknown.
Most problematic has been the activation of 5-HT
2B receptors by nearly all psychedelic drugs and the entactogen MDMA (
72,
78). For many years it has been known that drugs with potent 5-HT
2B agonist activity induce valvular heart disease in humans (
79)—for instance, fenfluramine, which was withdrawn from the market due to drug-induced valvular heart disease in as many as 30% of individuals (
80). This was subsequently demonstrated to be due to activation of the 5-HT
2B receptor by norfenfluramine, the major metabolite of fenfluramine (
81–
83). Subsequently, chronic treatment with several ergot derivatives used in treating Parkinson’s disease (
84,
85) as well as MDMA (
86) were associated with clinically significant valvular heart disease. Finally, ergot derivatives used for treating migraine headache have also been associated with valvular heart disease (
87). For drugs like ergotamine, this is due to the main metabolite, methylergonovine, which is a potent 5-HT
2B agonist (
81,
88,
89).
No studies have yet directly addressed the concern that chronic administration of psychedelic drugs—as might occur with “microdosing”—might induce clinically significant valvular heart disease. Such studies would likely require large numbers of subjects, assessed in a prospective fashion, as was done for the drug lorcaserin (
90,
91). Until such time as definitive studies are performed, we must caution against the long-term use of psychedelic drugs and MDMA.
Psychedelic and Psychedelic-Inspired Medications
Since the 1950s and 1960s, there has been evidence that psychedelic drugs might be useful for treating a variety of neuropsychiatric diseases (
6,
92), although this area of study was not without controversy (
93,
94). Over the decades since, there have been scattered and anecdotal reports of beneficial actions of psychedelics in obsessive-compulsive disorder (
70,
95), migraine (
96), cluster headaches (
97,
98), and other conditions (
3).
More recently, phase 2 placebo-controlled trials have reported significant actions of both single and two doses of psilocybin to rapidly reduce symptoms of depression and anxiety (
9,
10,
12,
13). A similar phase 2 placebo-controlled trial showed similar significant effects of LSD on depression and anxiety (
14). All these trials employed psychotherapy as part of the treatment regimen, and it is unknown to what extent therapist interventions might be key to the potential therapeutic actions of psychedelics (for recent perspectives, see references
3,
18).
Many approved antidepressant medications—including virtually all the tricyclic drugs (
99) as well as newer medications such as mirtazapine (
100) and brexpiprazole as adjunctive therapy (
101)—are potent 5-HT
2A antagonists. Related to this, it has long been known that chronic treatment with many antidepressant medications induces a downregulation of 5-HT
2A receptors (
102–
105). Intriguingly, both atypical antipsychotic drugs and psychedelics also can induce rapid downregulation of 5-HT
2A receptors (
106–
108). Collectively, these findings suggest that nonpsychedelic 5-HT
2A-active medications can function as therapeutic drugs for a variety of neuropsychiatric conditions (Gumpper and Roth, in press).
Given this background, recent studies have addressed the hypothesis as to whether it is possible to identify 5-HT
2A agonists that are not psychedelic and are potentially therapeutic (
3). To date, three groups have identified new drug-like molecules that—in mice—are devoid of psychedelic drug–like actions and have antidepressant-like actions (
109–
111). In all these instances, the new 5-HT
2A agonists displayed minimal activity at typical mouse models of psychedelic drug actions (e.g.,
110). Simultaneously, these drug-like molecules displayed robust antidepressant drug–like actions in a variety of rodent models (
109–
111). Although none of these molecules has advanced to clinical trials, these results support the hypothesis, and it will be informative to determine their effects in humans with depression and related disorders.