Suicide is a leading cause of death among Americans (
1,
2), with rates of suicidal thoughts and attempts differing markedly by biological sex. Females (assigned female at birth) are three times more likely than individuals assigned male at birth to report suicidal ideation, suicidal planning, or a suicide attempt (
3,
4). Thus, understanding suicide risk factors unique to females is critical to addressing this public health crisis.
Suicide research typically focuses on which biopsychosocial traits increase suicide risk. Measures of psychiatric symptoms, such as depression, hopelessness, anhedonia (
5–
7), perceived burdensomeness (
8), worthlessness (
9), rejection sensitivity (
10), agitation (
11), anxiety, and anger (
6), taken at single time points are well-established correlates of trait suicide risk. Although these studies have begun to clarify who is most vulnerable to suicidal ideation and attempts, questions of when and how suicide risk increases remain largely unanswered (
12).
The limited work investigating time-varying predictors has linked within-person increases in depression and stress with increased suicidal ideation (
13). Momentary experiences of sadness, worthlessness, feeling overwhelmed, anger, anxiety, and perceived rejection (
14), in addition to contextual factors such as interpersonal conflict and economic difficulty (
15), are linked with increased risk of suicide. Although hopelessness, anxiety, and anhedonia predict suicide attempts and deaths over the next year and beyond (
16), short-term risk factors that increase suicidal planning among individuals already experiencing suicidal ideation remain unknown.
Preliminary longitudinal studies have highlighted the co-occurrence of suicidal ideation and planning with stressors and related affective states; however, knowledge gaps remain in two key areas: the short-term risk factors that increase imminent suicide risk (e.g., planning a suicide attempt) among individuals with ongoing suicidal ideation, and when individuals are most vulnerable to states that correlate with increased suicidality. Consideration of more predictable, recurring biological and environmental factors may enhance our ability to answer the critical clinical question of when and why suicide risk fluctuates within patients broadly at risk for suicide.
To investigate time-variant suicide risk factors beyond the usual suspects of psychopathology and sociodemographic variables (
13,
17), we explored the menstrual cycle as a biological factor influencing acute suicide risk. The menstrual cycle is anchored by menses (day 1) and ovulation (day 14), with predictable fluctuations in estradiol and progesterone levels that hormonally trigger the follicular (menses to ovulation) and luteal (ovulation to next menses) phases (for an overview, see Figure S1 in the
online supplement). Accumulating evidence shows that ovarian hormone fluctuations modulate imminent suicide risk for many females, resulting in increased likelihood of suicide attempts and deaths during the premenstrual and menstrual weeks (
18–
21). However, the sparse work that has been done in this area is limited by small samples, cross-sectional data, assessment of menstrual cycle phase by asking when the last menses occurred (despite evidence that retrospective accounts of menses are often inaccurate [
19,
22,
23]), failure to distinguish chronic suicidal ideation from acute suicidal planning, and not taking into account individual differences in response to the cycle. Moreover, previous work has yet to clarify the psychosocial pathways by which the perimenstrual phase of the cycle increases suicide risk.
Many symptoms associated with suicide risk arise or worsen perimenstrually in a subset of individuals with a neurobiological sensitivity to the normal hormonal changes of the menstrual cycle (“hormone sensitivity”) (
24). Two well-established manifestations of hormone sensitivity are premenstrual dysphoric disorder (PMDD), a disorder characterized by intense, luteal phase–confined hormone sensitivity (
25), and premenstrual exacerbation, a worsening of the symptoms of a chronic psychiatric disorder during the midluteal or perimenstrual phase (
26,
27). The most common PMDD symptoms, irritability and anger, are established suicide risk factors (
6,
14). Furthermore, individuals with premenstrual dysphoric disorder report high rates of lifetime suicidal thoughts and behavior: roughly 72% of these individuals report active suicidal ideation, and approximately 34% report a history of suicide attempt (
28). Research shows that hormone-sensitive females with premenstrual exacerbation of depressive disorders experience midluteal and perimenstrual worsening of symptoms that correlate with suicidal thoughts and behaviors, such as depression, anhedonia, worthlessness, and guilt (
29), whereas hormone-sensitive females with premenstrual exacerbation of borderline personality disorder experience guilt, shame, rejection sensitivity, hopelessness, conflict, anxiety, and anhedonia (
30). Together, the previous evidence indicates that ovarian hormone fluctuations, specifically among hormone-sensitive females, increase a variety of symptoms linked to suicide risk and that these symptoms, as well as suicidal ideation and suicide risk, peak around menstruation (
18). However, to our knowledge, no research has been conducted to evaluate how daily changes in psychiatric symptoms covary with—or mediate changes in—suicidality across the menstrual cycle.
This study represents the first and largest longitudinal data set of its kind, utilizing intensive, daily symptom reports and gold-standard methods for the determination of menstrual cycle phase to empirically test psychosocial mechanisms of suicide risk across the menstrual cycle in a large transdiagnostic sample of females experiencing suicidality. We aimed to elucidate between- and within-person correlations between psychiatric symptoms and suicidality, relationships between the menstrual cycle and daily changes in symptoms and suicidality, and how daily symptom fluctuations longitudinally mediate the menstrual cycle–suicidality relationship. To account for differences in hormone sensitivity, we tested random slopes in all models, given that the cycle predicts psychiatric symptoms and suicidality to varying degrees across individuals. We hypothesized that the psychiatric symptoms selected for study (depression, hopelessness, anxiety, feeling overwhelmed, agitation, anhedonia, worthlessness, rejection sensitivity, anger, perceived burdensomeness, and interpersonal conflict), which are all known correlates of suicide, will positively correlate with suicidal ideation and planning both between and within participants. Moreover, we expected that, on average, the individuals in this sample would experience increases in symptoms and suicidality across the menstrual cycle, with symptoms being highest in the perimenstrual phase and lowest in the periovulatory phase, and that there will be variability in these effects across participants. Finally, we expected that associations between menstrual cycle phase and suicidal ideation and planning would be mediated by daily symptom fluctuation, although we did not make specific predictions about the relative predictive power of each mediator given the limited work in this area.
Methods
All planned analyses and hypotheses were preregistered on Open Science Framework (
https://osf.io/39m2x) prior to analysis.
Recruitment, Enrollment, and Procedures
Participants were recruited for parent clinical trials via social media and screened for eligibility as described on ClinicalTrials.gov (NCT03720847, NCT03498313, and NCT04112368). Relevant criteria included the following: assigned female at birth; ages 18–45; 21–35 day menstrual cycle; ongoing outpatient mental health care; not pregnant, no past-year childbirth, and not seeking to become pregnant; no hormonal medications or intrauterine device; no chronic physical conditions; no current nicotine use; not diagnosed as having PMDD (prospective diagnosis; exclusionary because of the hormonal manipulation in parent trial); no substance misuse that would interfere with study activities; endorsement of past-month suicidal ideation; and no evidence of concrete suicide planning with intent to act that would require immediate inpatient care. Participants provided written informed consent after the research team provided a full review of the study description and confirmed eligibility.
Participants completed daily electronic surveys that included self-report of menses onset and psychiatric symptoms (including suicidal ideation and planning) for at least one full menstrual cycle (“baseline”) prior to the experimental stages of the parent trials. Participants also completed a “washout” cycle between experimental phases, during which they continued to provide daily ratings with no experimental manipulation. The present analyses included daily ratings from only the baseline and washout cycles.
Measures
Cycle day and phase coding.
Using menses self-report (where menses is day 1) and ovulation testing based on the urine luteinizing hormone (LH) surge (where ovulation is day 0), we identified five phases per cycle, for each participant (
31): early luteal (days +2 to +5 after ovulation), midluteal (days −9 to −5 before menses), perimenstrual (days −3 to +2 surrounding menses), midfollicular (days −7 to −3 before ovulation), and periovulatory (days −2 to +1 surrounding ovulation) phases. A detailed description of phase coding procedures can be found in recently published work on alcohol use across the menstrual cycle (
32) and is included in the Methods section in the
online supplement. Any daily rating completed on a day that did not fall into a predefined phase was excluded from the cycle phase analyses. Per Schmalenberger et al. (
31), we present graphs of person-centered outcomes across all cycle days to provide a holistic view of symptom trajectories across the cycle.
Daily symptoms.
Surveys included a subset of items from the Daily Rating of Severity of Problems (
33) and the Adult Suicidal Ideation Questionnaire (
34), to capture suicidal ideation and suicidal planning, and one item each from the Interpersonal Needs Questionnaire (
35) and the Brief Agitation Measure (
36), to measure perceived burdensomeness and agitation, respectively. Symptoms were rated on Likert scales from “not at all” to “extremely” within the past 24 hours. See Table S1 in the
online supplement for a full list of daily-symptom Likert scales and the calculation of the suicidal ideation and planning variables. Participants also provided daily menses and ovulation testing data. For between-person indices of symptoms, we calculated and z-scored a cluster mean per participant. For within-person indices of symptoms, we calculated daily deviations from the person-centered mean by subtracting that person’s mean value from their symptom rating for that day.
Analytic Plan
Multilevel structural equation models were conducted in Mplus, version 8.8, with maximum likelihood as the default estimator and full information maximum likelihood as the default treatment of missing values.
Aim 1: Psychiatric symptoms as predictors of suicidal ideation and suicidal planning.
Our first set of multilevel structural equation models examined symptoms as predictors of suicidal ideation (linear regression) and suicidal planning (logistic regression). Daily observations (level 1; N=6,018) were nested within participants (level 2; N=119). In both models, all psychiatric symptoms were entered simultaneously for the between- and within-person analyses. For each within-person predictor, random slopes (i.e., the association between daily symptom and suicidal ideation or suicidal planning) were modeled as latent variables to quantify individual differences in associations between symptoms and either suicidal ideation or suicidal planning.
Aim 2: Menstrual cycle phases as predictors of daily changes in psychiatric symptoms, suicidal ideation, and suicidal planning.
Our next set of multilevel structural equation models tested menstrual cycle phase contrasts as predictors of daily changes in psychiatric symptoms (linear regression), suicidal ideation (linear regression), and suicidal planning (logistic regression). We tested pairwise comparisons between specified cycle phases for each outcome and included four models per outcome to rotate the reference group (periovulatory, perimenstrual, early luteal, or midluteal). Cycle phase was a dummy-coded categorical variable, such that five binary variables indicated whether a day fell in a particular phase, allowing four contrasts per model. Random intercepts and slopes for each phase contrast were modeled as latent variables to quantify individual differences in cycle-symptom relationships (i.e., differences in hormone sensitivity).
Aim 3: Psychiatric symptoms as mediators of the relationship between menstrual cycle phase and suicidal ideation and planning.
Our final set of multilevel structural equation models tested all daily symptoms simultaneously as mediators of the relationship between menstrual cycle phase contrasts and suicidal ideation and suicidal planning; random slopes for each path were modeled as latent variables, and indirect effects of daily symptoms on suicidal ideation and suicidal planning were computed. Following current recommendations for multilevel structural equation mediation modeling (
37,
38) and mediation with binary outcomes (
39), each indirect effect for each daily symptom was computed by multiplying two regression coefficients. The first coefficient represents the association of menstrual cycle phase with a daily symptom (e.g., depression). The second coefficient represents the association of the daily symptom with an outcome (i.e., suicidal ideation or suicidal planning).
Results
Participants
Our transdiagnostic sample included 119 naturally cycling females, resulting in 6,018 observations (13–158 observations per person). The total number of observations varied between individuals as a result of excluding observations outside of specified cycle phases, and continuing to collect daily ratings when a parent trial was halted under COVID-19 restrictions, which resulted in extended baselines. The mean age was 28.4 years (SD=6.7); 94.1% of participants identified as women (5.9% non-binary, genderfluid, or genderqueer); and our sample was 50.4% heterosexual, 26.1% bisexual, 10.9% gay or lesbian, and 12.6% other, queer, asexual, questioning, or exploring. Participants reported the following races/ethnicities (participants could select multiple races): 66.4% Caucasian, 10.9% Black/African American, 17.6% Asian, 1.7% American Indian/Alaskan Native, and 8.4% other, unknown, or declined to answer; 16.0% reported Hispanic/Latinx ethnicity. The highest level of education achieved by participants included earning a bachelor’s degree (37.0%), completing postgraduate education (26.9%), completing some college, associate’s degree requirements, or trade school (26.1%), earning a high school diploma (8.4%), and completing some high school (0.8%). On the basis of the research version of the Structured Clinical Interview for DSM-5 (
40), administered by licensed clinical psychologists or their supervised graduate students, participants met DSM-5 criteria for the following diagnoses: depressive disorders (73.0%), anxiety disorders (65.6%), posttraumatic stress disorder (30.3%), attention deficit hyperactivity disorder (17.6%), any substance use disorder (10.1%), and any eating disorder (10.1%). Approximately 53% of the sample were using antidepressants (e.g., selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, or bupropion) stably throughout the study. Using the Carolina Premenstrual Assessment Scoring System (C-PASS) to conduct cycle-level diagnosis in at least one baseline cycle (
41), we found that 41.2% of participants showed a premenstrual elevation of at least 30% in one or more of the core emotional symptoms in at least one cycle, 26.8% met C-PASS criteria for menstrually related mood disorders in at least one cycle (PMDD pattern present for at least one core emotional symptom), and 0.9% met C-PASS criteria for PMDD in one cycle (PMDD pattern present for five or more symptoms, including one emotional symptom).
Aim 1: How Do Psychiatric Symptoms Covary With Suicidal Ideation and Planning?
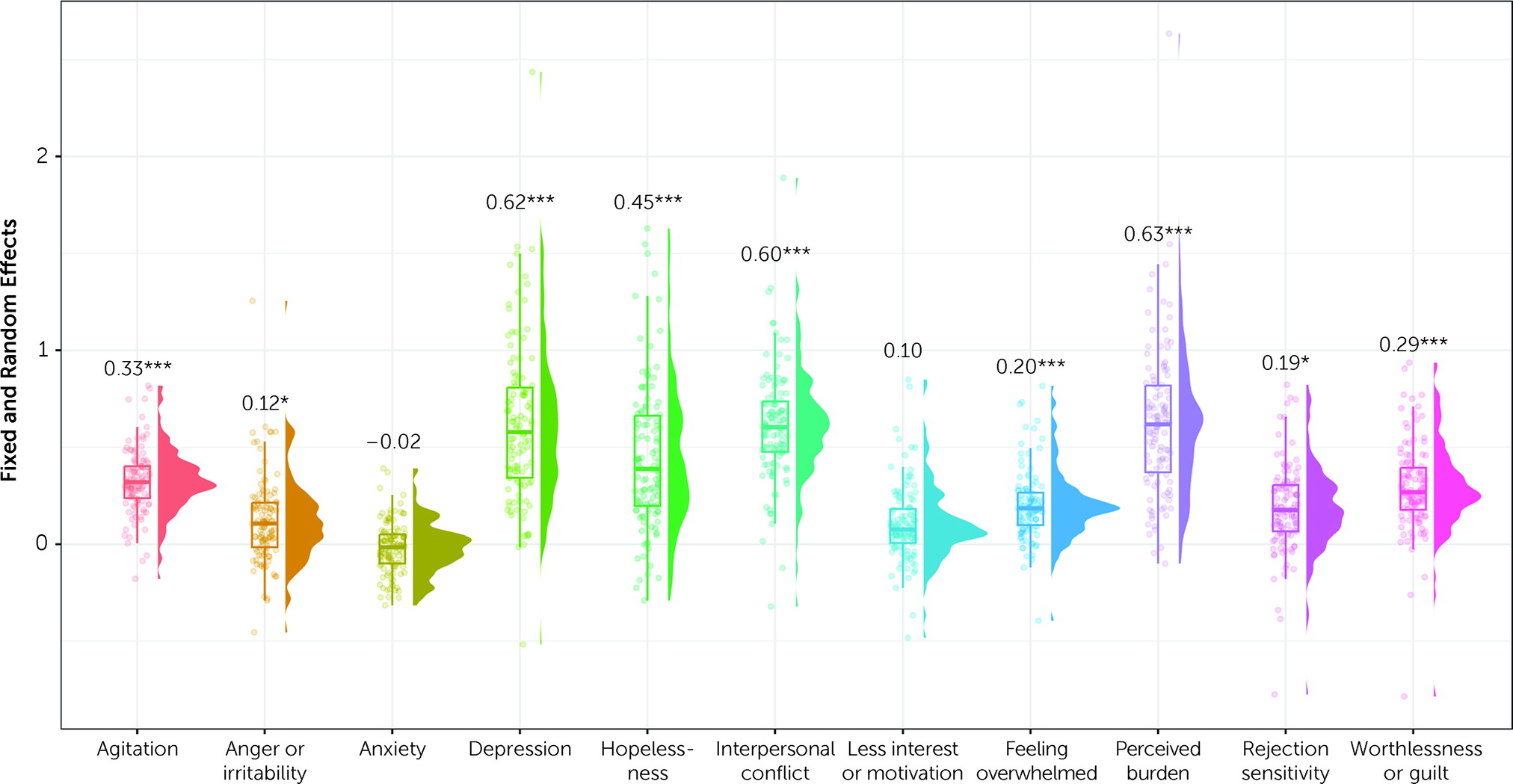
The results addressing aim 1 are shown in
Figure 1. Intraclass correlation coefficients from null models nested within participants with no predictors for suicidal ideation and suicidal planning suggested that stable between-person differences accounted for approximately half of the variance in suicidal ideation (intraclass correlation coefficient=0.53) and one-third of the variance for suicidal planning (intraclass correlation coefficient=0.31). A multilevel structural equation model testing associations between symptoms and suicidal ideation revealed that trait feelings of worthlessness (estimate=1.031, SE=0.415, p=0.013) and perceived burdensomeness (estimate=0.816, SE=0.303, p=0.007) uniquely predicted trait suicidal ideation.
Estimates of within-person effects (i.e., regression coefficients that reflect associations between predictor variables and suicidal ideation or suicidal planning) revealed that daily fluctuations in all symptoms except anxiety independently covaried with suicidal ideation (
Figure 1). All random effects (i.e., variances of the random effects for the specified random slopes in our model) were significant, suggesting individual variability in the covariation of symptoms and suicidal ideation.
Estimates of logistic regression coefficients (i.e., regression coefficients representing the log odds of the probability of suicidal planning as a linear combination of the predictor variables, as is the default in Mplus) revealed that only trait levels of feeling overwhelmed predicted increased trait likelihood of suicidal planning (estimate=0.975, SE=0.466, p=0.037). Within-person changes in depression (estimate=0.27, SE=0.092, p=0.003), hopelessness (estimate=0.217, SE=0.102, p=0.034), rejection sensitivity (estimate=0.174, SE=0.07, p=0.013), and perceived burdensomeness (estimate=0.199, SE=0.067, p=0.003) predicted greater day-to-day likelihood of suicidal planning. All other symptoms and random effects were nonsignificant (p>0.05 in each case), suggesting minimal person-to-person variability in associations between psychiatric symptoms and suicidal planning.
Aim 2: How Do Symptoms and Suicidal Ideation and Planning Vary Across the Menstrual Cycle?
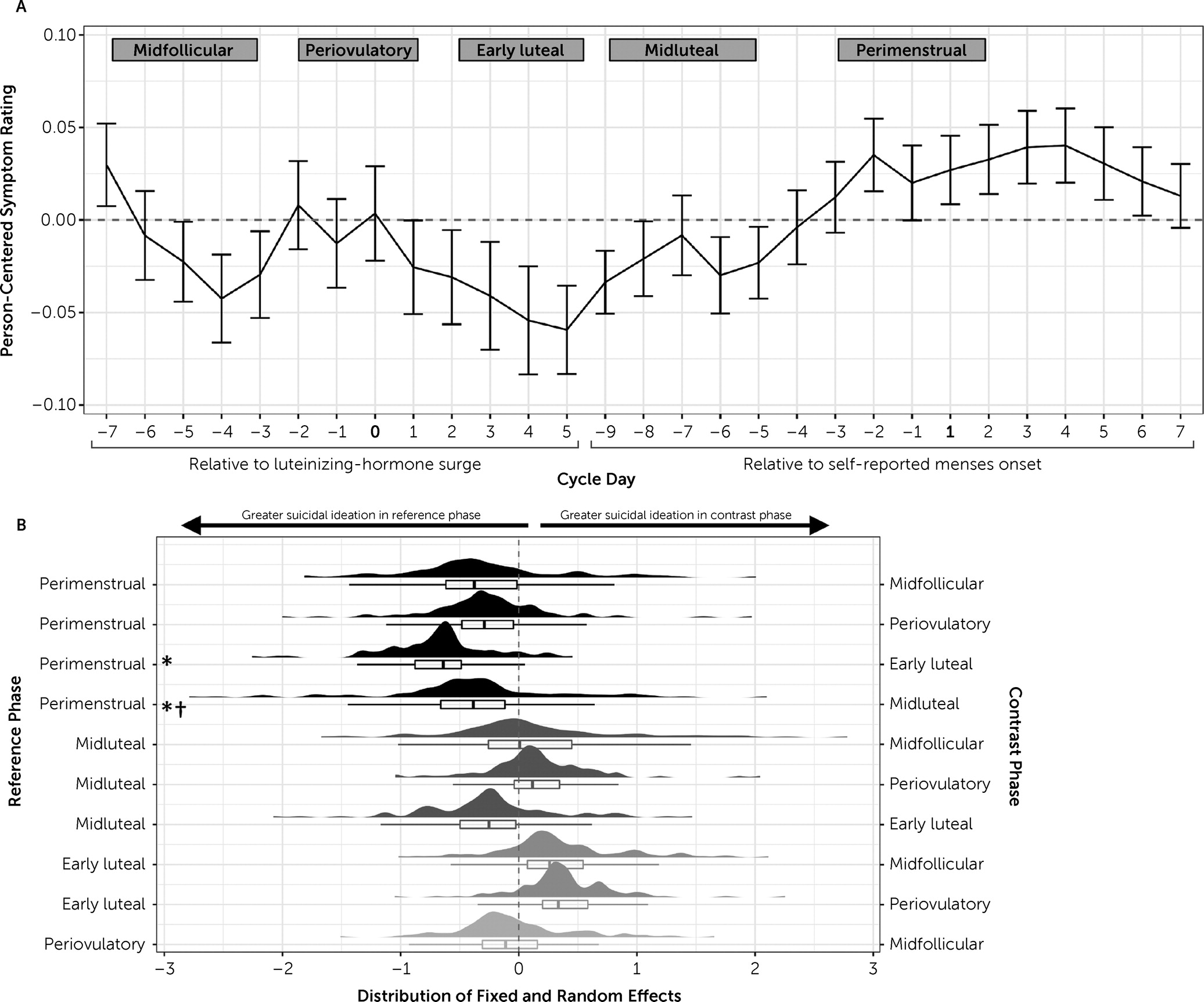
Results related to aim 2 are provided in
Table 1. Visualizations of symptom trajectories across the cycle and random effect distributions for suicidal ideation are provided in
Figure 2 (for all other symptoms, see Figures S2–S12 in the
online supplement). To broadly summarize, all psychiatric symptoms, except agitation, were most severe during the perimenstrual phase compared with at least one other phase, and symptoms during the early luteal phase were lowest in severity compared with at least one other phase. Symptom trajectories between the midluteal, periovulatory, and midfollicular phases varied by model, whereas perimenstrual peaks and early luteal troughs were found for all outcomes.
For most symptoms (depression, hopelessness, loss of interest, anxiety, worthlessness, rejection sensitivity, hopelessness, and interpersonal conflict), random effects of cycle phase were significant for at least one contrast. Only feeling overwhelmed, agitation, and perceived burdensomeness had nonsignificant random effects for all phase contrasts, suggesting that the fixed effects of cycle phase on these symptoms were uniform across individuals.
Suicidal ideation was more severe in the perimenstrual phase relative to early luteal or midluteal phases (
Figure 2). Suicidal planning was more likely to occur in the perimenstrual phase relative to midfollicular or early luteal phases and in the midluteal phase relative to early luteal phase. Random effects of cycle phase on suicidal ideation were significant for the perimenstrual-versus-midluteal contrast (estimate=1.34, SE=0.61, p=0.027), which suggests that individual differences in hormone-related suicidal ideation were most notable after ovulation through menses. All random effects in suicidal planning models were nonsignificant, suggesting that midluteal and perimenstrual worsening of suicidal planning were consistent across individuals in our sample. Taken together, the findings provide strong evidence of hormone-sensitive cyclical changes, but there were significant individual differences in hormone sensitivity.
Aim 3: Which Symptoms Mediate Cyclical Changes in Suicidal Ideation and Planning?
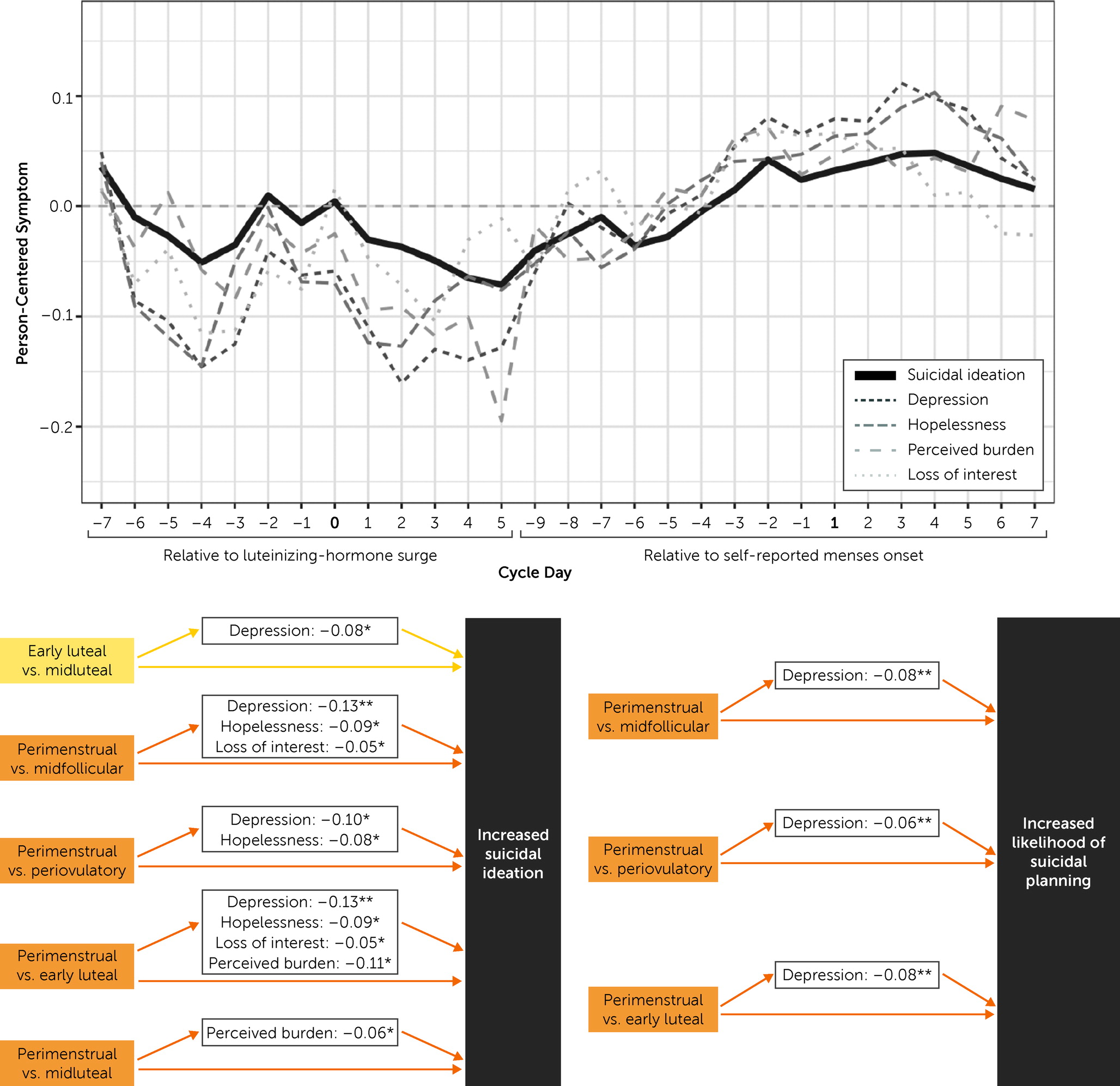
Our final set of models tested mediation hypotheses to understand which daily symptoms account for cyclical changes in suicidal ideation and suicidal planning. For pairwise cycle-phase contrasts in which suicidal ideation or suicidal planning was significantly different between phases, we analyzed indirect effects of each symptom in a single multiple mediation model (
Table 2,
Figure 3).
Depression and perceived burdensomeness mediated perimenstrual-versus-early-luteal and perimenstrual-versus-midluteal changes in suicidal ideation. Hopelessness and loss of interest mediated increased suicidal ideation from the early luteal phase to the perimenstrual phase, but these symptoms did not contribute to the midluteal to perimenstrual increase.
Depression mediated both the perimenstrual-versus-midfollicular and perimenstrual-versus-early-luteal increases in likelihood of suicidal planning. All other mediation effects on suicidal planning were nonsignificant.
Discussion
In this study, we aimed to extend previous work by 1) translating established between-person predictors of suicidal ideation to within-person predictors of acute suicidal ideation, 2) distinguishing suicidal ideation from planning, 3) clarifying the role of the menstrual cycle in daily fluctuations in symptoms and suicidality, 4) exploring which symptoms uniquely account for the relationship between menstrual cycle phase and suicidality, and 5) characterizing individual differences (e.g., hormone sensitivity) in psychiatric symptoms and suicidality across menstrual cycle phases.
How Do Psychiatric Symptoms Covary With Suicidal Ideation and Planning?
Our findings replicate established between- and within-person associations between psychiatric symptoms and suicidality (
5–
14). While worthlessness and perceived burdensomeness were salient predictors of severity of trait suicidal ideation, feeling overwhelmed was the only significant between-person predictor of suicidal planning. Therefore, at the between-person level, self-directed negative cognitions predicted severity of suicidal ideation, and a trait tendency toward perceptions of high stress and insufficient capacity to cope predicted suicidal planning. At the within-person level, nearly all symptoms, with the exception of anxiety and conflict, significantly co-fluctuated with suicidal ideation. Of note, every model had significant random effects for symptom–suicidal ideation associations, suggesting that attention to individual differences in vulnerability factors (e.g., degree of hormone sensitivity) is crucial for prevention efforts. Meanwhile, a variety of depressive symptoms accounted for changes in suicidal planning (depression, hopelessness, rejection sensitivity, and perceived burdensomeness). Thus, whereas a broad range of emotional and cognitive symptoms contributed to within-person variability in suicidal ideation (e.g., depressed mood, feeling overwhelmed, and anger), a limited set of uniquely depressive symptoms predicted increased likelihood of suicidal planning among individuals already experiencing suicidal ideation.
How Do Symptoms and Suicidal Ideation and Planning Vary Across the Menstrual Cycle?
Our findings indicate that females with ongoing suicidality appear to be at high risk for exacerbation of psychiatric symptoms and suicidality in the perimenstrual phase. Random effects suggest that symptom changes in response to the menstrual cycle vary significantly across people; this underscores the importance of understanding individual differences in hormone sensitivity (i.e., how people respond to normal fluctuations in ovarian hormone levels) when generalizing about cycle-related psychiatric risk. Previous cross-sectional studies have linked suicide risk with the perimenstrual weeks (
19) and with hormone sensitivity (e.g., PMDD diagnoses) (
28); we extend this area of research by providing the first study, to our knowledge, that tests these associations in a prospective manner, with daily data collection and gold-standard urine luteinizing hormone testing for cycle-phase measurements. Critically, our analyses show that even when patients do not meet the strict diagnostic criteria for PMDD, a large subset of females with suicidality experience hormone sensitivity, or premenstrual exacerbation of their symptoms, and suicidal ideation.
Which Symptoms Mediate Cyclical Changes in Suicidal Ideation and Planning?
Depression, perceived burdensomeness, hopelessness, and anhedonia emerged as the most robust mediators of perimenstrual worsening of suicidal ideation. However, depressed mood was the only symptom that significantly mediated perimenstrual worsening of suicidal planning over and above all other symptoms. These findings suggest once again that depressive symptoms play a salient role in perimenstrual exacerbation of suicidality, underscoring the need for research that identifies the pathophysiology of cyclical changes in depressive symptoms to understand and prevent cyclical worsening of suicidality (
42).
Strengths and Limitations
This study has several strengths. First, whereas most suicide research highlights broad psychopathological and sociodemographic factors as predictors of suicide risk, our study reveals an acute perimenstrual risk period for suicidal ideation and planning, presenting an opportunity for biological and psychosocial intervention among at-risk females. Second, the few studies that have investigated menstrual cycle and suicidal thoughts and behaviors primarily used cross-sectional data, which limits the capacity to understand fluctuations in suicidality across time and, consequently, to predict when individuals are most at risk. A key strength of the present study is the prospective, longitudinal rating of symptoms. Third, our protocol included gold-standard methods for cycle-phase designations, combining urine luteinizing hormone surge testing with menses self-report. Finally, we relied on a large sample (N=119) with recent suicidal ideation, allowing for a well-powered, transdiagnostic investigation of how psychiatric symptoms change across the menstrual cycle and indirectly account for cyclical changes in suicidal ideation and planning.
However, this study is not without limitations. First, our sample was not recruited for any specific hormone sensitivity patterns, and the participants did not meet criteria for PMDD (this was intentional, because of the primary endpoints of the parent clinical trials); thus, the pattern of results might be different in samples of individuals who were recruited for hormone sensitivity, specifically, premenstrual exacerbation of suicidal ideation or luteal phase–confined PMDD. Second, we did not test for time-lag effects (e.g., effects of previous-day symptoms on the prediction of next-day suicidal ideation and planning) because we did not have hypotheses for a specific time lag and because temporal associations between psychiatric symptoms and suicide risk may occur at shorter intervals than the daily ratings available with our data; future research may benefit from more frequent assessments per day using ecological momentary assessment methods. Additionally, given the rarefied nature of the data set and the limited previous research on this topic, we prioritized control of type II errors (false negatives) and chose not to control for multiple tests. This approach necessarily increases the risk of type I errors (false positives) and increases the importance of independent replication. Finally, the analyses focused on phase-level clustering of symptoms and random slopes, both of which complicate attempts to account for autocorrelation. Methodological work is needed to determine best practices in this area.
Conclusions
This study replicates findings from previous cross-sectional studies showing increased suicide risk during the perimenstrual phase (
19) and extends this area of research by demonstrating how the relationships between suicidality and symptoms fluctuate daily within individuals. Our results suggest that among females with suicidality, levels of suicidal ideation, suicidal planning, and nearly all measured symptoms (i.e., hopelessness, anxiety, depression, interpersonal conflict, anhedonia, feeling overwhelmed, perceived burdensomeness, rejection sensitivity, and worthlessness) peak during perimenstrual days. We also found significant random effects in most models, providing evidence that although there are average relationships between the cycle and symptom fluctuations, these associations are highly variable between people—we urge researchers to model individual symptom trajectories in hopes of understanding subtypes of neurobehavioral hormone sensitivity (
43). Moreover, this work suggests that depressive symptoms (i.e., depressed mood, hopelessness, and anhedonia) are robust mediators of perimenstrual worsening of suicidal ideation and planning. These findings highlight the need for more intensive, longitudinal research on the mechanisms underlying and the prevention of suicidality across the cycle. We conclude that intervention efforts may benefit from strategies that specifically target depressive symptoms as they fluctuate across the cycle, given their powerful role in the cycle-suicidality relationship.