Use of levonorgestrel-releasing intrauterine systems (LNG-IUSs) has been associated with an approximately 40% increased risk of developing depression and initiating antidepressant medication (
1–
3). An increased risk of depression has also been observed when LNG-IUS users were compared with users of non-hormonal (copper) IUSs (
4). However, the literature on mood symptoms related to LNG-IUS use has also shown mixed results (
5). Most of the current literature is limited by the observational nature of studies and by vulnerability to confounding, including a healthy user bias (
6), which may explain the conflicting results in the literature. Therefore, research is needed that addresses the potential relationship between exogenous hormone exposure from LNG-IUSs and risk of developing depression.
LNG-IUSs are available in over 120 countries worldwide, and they are used by about 14% of women of reproductive age in Denmark, equivalent to one in three hormonal contraceptive users (
7,
8). Today, LNG-IUSs are available in three formulations, differentiated by the total LNG content and daily release dose: the low-dose LNG-IUS contains 13.5 mg, with a 14 μg/day initial release dosage and an 8 μg/day average over the first year (
9); the medium-dose LNG-IUS contains 19.5 mg, with a 17.5 μg/day initial release dosage and a 12.6 μg/day average over the first year (
10); and the high-dose LNG-IUS contains 52 mg, with a 21 μg/day initial release dosage and a 20 μg/day average over the first year (
11). All three types are used as birth control, and the high-dose LNG-IUS is also approved for treating heavy menstrual bleeding (menorrhagia) (
11). The mechanism of action of LNG-IUS as a contraceptive method is exerted via local effects in the uterus by inducing endometrial thinning and increased cervical mucus, which inhibits sperm survival and prevents fertilization and implementation of the oocyte (
12). However, among users of LNG-IUSs, only about 50%–76.5%, 88%, and 97% of high-, medium-, and low-dose users, respectively, have ovulation during the first year after insertion; the rates increase to about 80%–100% the following year, which indicates some systemic effects of LNG-IUS, particularly early after insertion (
13–
15). Such effects are in line with a higher daily release dose of LNG during the early treatment phase, which declines with duration of use; the daily release doses decline to 6 μg/day, 10 μg/day, and 19 μg/day for low-, medium-, and high-dose LNG-IUSs, respectively, 1 year after insertion (
9–
11). LNG is able to inhibit ovulation because it is a potent progesterone receptor agonist, by which it can mimic endogenous progesterone effects and provide negative feedback to the hypothalamic-pituitary-gonadal axis (
16). However, it also activates other receptors, including androgen, glucocorticoid, and mineralocorticoid receptors, with the potential to affect brain biology (
17).
Results
The study population included 149,200 first-time LNG-IUS users (see Figure S1 in the
online supplement), of whom 22,029 (14.8%) used low-dose, 47,712 (32.0%) medium-dose, and 79,459 (53.3%) high-dose LNG-IUSs (
Table 1). First-time users of low-dose LNG-IUSs were younger on average (mean age, 22.9 years [SD=4.5]) than first-time users of medium-dose (mean age, 25.2 years [SD=6.2]) and high-dose LNG-IUSs (mean age, 30.2 years [SD=5.6]). Correspondingly, their education level was lower on average, and they were more often nulliparous (89.7%, 69.8%, and 17.9% for the low-, medium-, and high-dose groups, respectively). Compared with first-time users of low-dose LNG-IUSs, high-dose LNG-IUS users were more often diagnosed with potential medical indications for LNG-IUS use, but this was not the case for medium-dose LNG-IUS, except for the diagnosis of polycystic ovary syndrome. Notably, rates of having menorrhagia as a prescription indication were 3.4%, 6.1%, and 10.9% in the low-, medium-, and high-dose LNG-IUS groups.
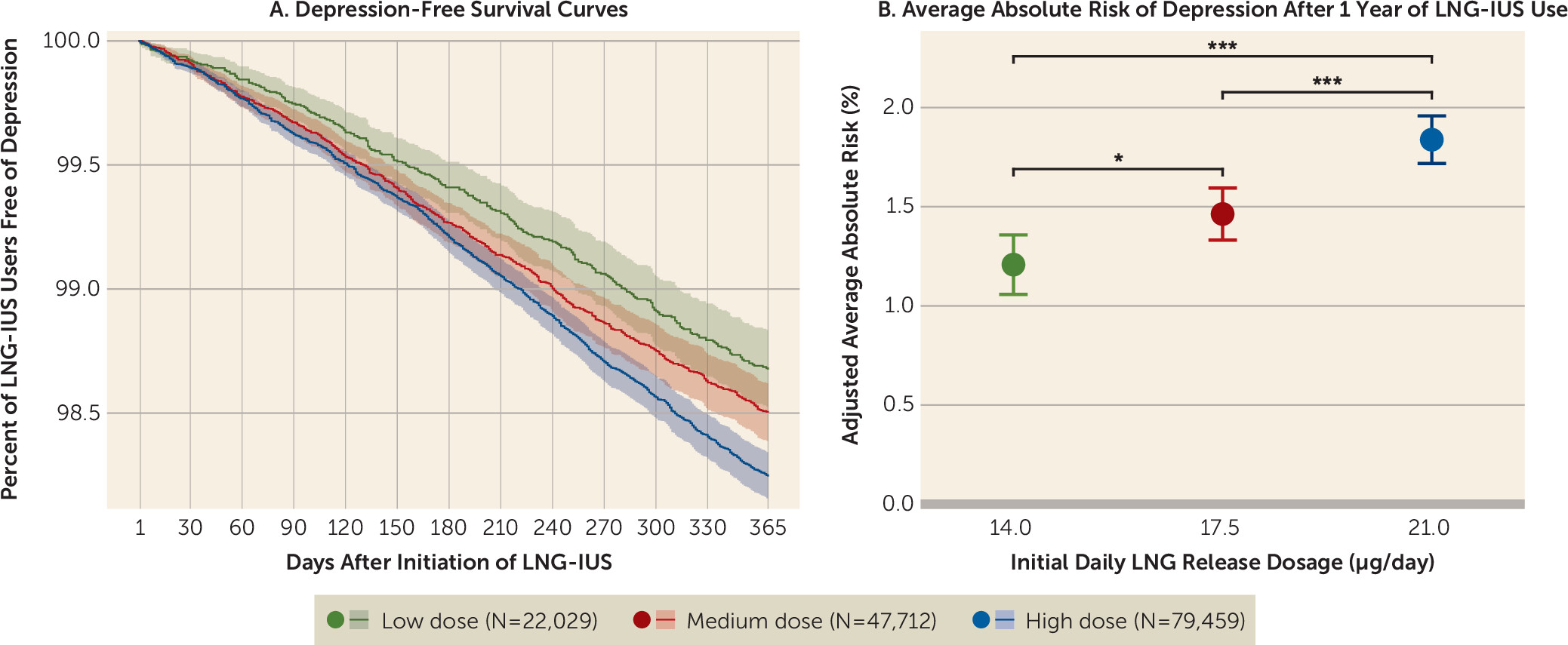
Within 12 months after initiating low-dose LNG-IUS use, 279 women were registered with incident depression, compared with 633 among medium-dose users and 1,346 among high-dose users. This corresponded to adjusted absolute risks of 1.21% (95% CI=1.06–1.36), 1.46% (95% CI=1.33–1.59), and 1.84% (95% CI=1.72–1.96), respectively. The risk ratios of developing depression for high-dose LNG-IUS use were 1.52 (95% CI=1.30–1.74) and 1.26 (95% CI=1.10–1.41) compared with low-dose and medium-dose LNG-IUS use, respectively. The risk ratio for medium-dose LNG-IUS use was 1.21 (95% CI=1.03–1.39) compared with low-dose LNG-IUS use (
Table 2,
Figure 1A–B). The estimated hazard ratios for the complete Cox proportional hazard model are shown in Figure S2 in the
online supplement. Similar trends were found when the outcome was analyzed as incident antidepressant use and depression diagnosis, separately (see Table S2 in the
online supplement). However, given the lower number of depression diagnoses, these risks were estimated with larger uncertainty. When prescriptions for antidepressant use with indications other than depression were excluded, the estimates supported the main finding (see Table S3 in the
online supplement).
When the population was restricted to first-time users between 2017 and 2022 (for demographic and clinical characteristics, see Table S4 in the online supplement), the risk ratios were 1.37 (95% CI=1.10–1.64) and 1.17 (95% CI=1.01–1.34) between high-dose and low-dose and between high-dose and medium-dose LNG-IUS use, respectively (see Table S5 in the online supplement). When the population was restricted to nulliparous first-time users under age 30, the groups were more comparable in terms of education level and age, with mean ages of 21.7 years [SD=3.3], 21.7 years [SD=3.2], and 22.3 years [SD=3.3] for low-, medium-, and high-dose LNG-IUS users, respectively (see Table S6 in the online supplement). The results based on this population (see Table S7 in the online supplement) were also in line with the main analysis, with risk ratios of 1.42 (95% CI=1.16–1.68) and 1.21 (95% CI=0.98–1.44) between high-dose and low-dose and between high-dose and medium-dose LNG-IUS use, respectively. When menorrhagia as prescription indication and previous hormonal contraceptive use were adjusted for, the risk ratios were 1.50 (95% CI=1.28–1.71) and 1.24 (95% CI=1.08–1.40) between high-dose and low-dose and between high-dose and medium-dose LNG-IUS users, respectively (see Table S8 in the online supplement). The risk ratios of depression due to menorrhagia as diagnosis and prescription indication were 1.52 (95% CI=1.20–1.85) and 1.19 (95% CI=1.02–1.36), respectively. Based on the estimated risk ratios from the main analysis, E-values were estimated at 2.41 (with lower border of 1.92) and 1.71 (with lower border of 1.21) for the association of depression in high- and medium-dose compared with low-dose LNG-IUS users, respectively. The propensity score–weighted analysis, which showed balanced standardized mean differences of covariates (see Table S9 in the online supplement) and overlapping propensity score distributions (see Figure S3 in the online supplement), showed risks comparable to those obtained with the G-formula: 1.16%, 1.47%, and 1.62% in absolute risk in low-, medium-, and high-dose LNG-IUS users and hazard ratios of 1.40 (95% CI=1.10–1.77) and 1.27 (95% CI=1.02–1.59) for high- and medium-dose compared with low-dose LNG-IUS users. In the final sensitivity analysis on women with prior mental disorders (N=48,937), the absolute risks were 4.80%, 5.22%, and 5.91% for low-, medium-, and high-dose LNG-IUS users, respectively, with risk ratios of 1.23 (95% CI=1.07–1.40) and 1.13 (95% CI=1.01–1.25) for high-dose compared with low- and medium-dose LNG-IUS users (see Table S10 in the online supplement).
Discussion
This population-based cohort study demonstrated a positive association between LNG dosage and risk of incident depression across initial LNG release dosages of 14 μg/day, 17.5 μg/day, and 21 μg/day. Notably, the 1-year absolute risks of depression were relatively small, at 1.21%, 1.46%, and 1.84% in first-time users of low-, medium-, and high-dose LNG-IUSs, respectively.
These results are in line with findings from an international multicenter randomized study linking LNG exposure and risk of developing depressive symptoms (
32). In that study, a higher frequency of self-reported depressive symptoms in the initial treatment phase and higher rates of discontinuation reported as being due to depression were observed among women allocated to LNG-IUS compared with women allocated to copper IUS. Furthermore, our results are consistent with those from a recent observational study (
18) that showed a 13% higher risk of antidepressant use among high-dose compared with medium-dose LNG-IUS users.
A recent study comparing the incidence of starting antidepressant medication associated with first-time use of the three different dosages of LNG-IUS in a population restricted to nulliparous women (
33) similarly showed an association with increased 1-year risk among high-dose (34.8 per 1000 person-years) compared with medium-dose (18.6 per 1000 person-years) and low-dose (14.0 per 1000 person-years) LNG-IUS users. However, the study found no statistically significant difference between low- and medium-dose LNG-IUS users, which was explained by a lack of difference in LNG plasma levels among users of these two LNG-IUSs. This explanation, however, is not supported by studies demonstrating that hormone exposure increases with increasing release dosage (
15,
34). The present study excluded women who had prior mental disorders or had ever used a psychotropic drug. Prior mental disorders appeared to attenuate the estimated relative risk, despite the presence of absolute differences, but these were not sufficiently large to follow the relative higher absolute risk in this population. This observation has also been reported in other studies (
4,
35). It does not necessarily contradict the notion that having a prior mental disorder increases the likelihood of experiencing mood symptoms after initiation of hormonal contraception (
36). However, the relatively lower risk associated with hormonal contraceptive use or with the higher LNG-IUD dosage in the high-risk population could be explained by a lower representation of the more hormone-susceptible population when different etiologies are likely to be highly represented. Alternatively, it could also be explained by a potential selection bias if women with prior mental disorders are more likely to prefer lower hormone exposure because of concern about the potential mood side effects.
Intriguingly, our study provides evidence of a dose-dependent association between LNG exposure and risk of subsequent depression across three dosages, which was consistent after considering potential confounders, such as menstrual bleeding indications for high-dose LNG-IUS use. As addressed in a recent meta-analysis (
37), the role of the hormonal contraceptive formulation in mood symptoms has been inconclusive; however, the present findings add new evidence on the potential role of the progestogen component of hormonal contraception in the development of mood disorders (
38). The knowledge on how progestogens affect the brain and mood and whether the route of administration plays a role is still limited (
5). Some evidence points to a potential mechanism through modulating effects on the regulation of stress reactivity via the hypothalamic-pituitary-adrenal axis (
39), and preclinical evidence points to a link between LNG-induced alterations in brain γ-aminobutyric acid receptor plasticity and anxiety-like behavior in rats (
40).
Even though the 21% and 52% higher relative risks associated with medium- and high-dose compared with low-dose LNG-IUSs amounted to absolute differences of only 0.27% and 0.66%, they highlight the clinical relevance of considering LNG hormone exposure dosage in contraceptive counseling, which may prove relevant together with information on prior reactions to hormonal contraceptives or postpartum depressive episodes (
41). These risk differences should be weighed against beneficial medical aspects, including better bleeding control when using high-dose LNG-IUS (
20), but also against possible negative outcomes, including an increased risk of ectopic pregnancy with lower LNG dosage (
42,
43). It also highlights the importance of educating women to be aware of potential mental health side effects and of clinical evaluation of mood symptoms at follow-up visits after LNG-IUS insertion.
The strengths of the present study include the use of national register data, enabling the study of an unselected nationwide population of first-time users of the different LNG-IUS dosages who had never used any form of psychotropic medication or had any prior mental disorders. Furthermore, it is a strength that the study compares the risk between users of different LNG-IUS dosages and not to a non-user population, which makes it less prone to a surveillance bias (
44) or behavioral confounders related the point of starting contraception (
45). Another strength is that the study used both prescriptions with information about prescription indication and depression diagnoses to best capture depressive episodes. Last, and importantly, the use of a relatively short follow-up period increases the likelihood that the event of starting an LNG-IUS and the event of depression could be causally linked, and it reduces the risk of healthy survivor bias and the number of women who discontinued within the follow-up period. Discontinuation most often goes undetected in the registers, yet, even if censoring at time of discontinuation had been possible, discontinuation could be due to mood symptoms, and thus it would be a violation of discontinuation being independent of the outcome.
The study also has limitations. First and foremost, an observational study does not allow direct causal inference, although the choice of a close temporal window between incident use of an LNG-IUS and registration of a depressive episode and the demonstration of a dose-response relationship jointly add indirect evidence supporting a causal relationship. Second, prescription patterns of antidepressants and LNG-IUSs changed during the study period, which may introduce bias; however, the sensitivity analysis restricting the study period to 2017–2022 supported the main finding. Third, first-time users of the different LNG-IUSs differed in age and parity, and higher age and recent delivery may introduce confounding by indication, as recent delivery and some chronic diseases would make women choose a progesterone-only contraceptive. However, the sensitivity analysis restricted to nulliparous first-time users under age 30, where the groups were more comparable in age, confirmed the main results. Fourth, potential confounders include undiagnosed medical conditions and unregistered off-label medical indications for the use of the high-dose LNG-IUS. However, women who had an LNG-IUS inserted at a hospital for treatment of a medical condition or who bought LNG-IUSs directly in a specialist clinic were not included in the study. Further, we did account for various diagnosed medical indications and menorrhagia as prescription indication, and as much as 4.5%, 7.3%, and 12.4% were known to have menorrhagia (diagnosed or via prescription indication) among first-time users of low-, medium-, and high-dose LNG-IUSs, respectively. In comparison, the prevalence of menorrhagia is estimated to be 9%–14%, although self-reported estimates reach 27% (
46,
47). Nonetheless, this had little impact on the results, which may be related to the fact that high-dose LNG-IUSs are effective in treating these medical disorders, resulting in improvement in quality of life (
48,
49). Additionally, based on the E-values, an unmeasured confounder should be associated with the exposure and the outcome with a strength of 2.41 and 1.71 to explain the increased risk associated with high- and medium-dose compared with low-dose LNG-IUSs, which is larger than the estimated risk ratios of menorrhagia as diagnosis and as prescription indication. This indicates that the confounding would need to be strong for the observed association to hold under the null hypothesis (i.e., a false positive result).
As hypothesized, this study provides evidence of a dose-dependent association between intrauterine levonorgestrel exposure and incident depression; that is, high-dose LNG-IUS use was associated with a 26% higher relative risk than medium-dose LNG-IUS use, which was associated with a 21% higher relative risk than low-dose LNG-IUS use. These differences remained after controlling for medical diagnoses and indications for prescribing high-dose LNG-IUSs. The 1-year absolute risks were 1.21%, 1.46%, and 1.84% for low-, medium-, and high-dose LNG-IUSs, emphasizing that increasing dosages were associated with only a small proportion of women developing depression to a degree that resulted in medical attention. These findings should be interpreted in light of the limitations of an observational study design with risk of residual confounding, and the observed risk differences should be weighed against potential benefits as well as other side effects of LNG-IUS use when providing personalized contraceptive counseling.