Studies of the treatment of major depressive disorder (MDD) rarely focus on patients whose illness has not responded to four or more antidepressant therapies (
1–
4). Such patients present with severe treatment-resistant depression (severe TRD). Serial antidepressant trials show diminishing success for patients unresponsive to initial treatment courses. In the STAR*D trial, remission rates were 36.8%, 30.6%, 13.7%, and 13.0% for the first, second, third and fourth interventions, while relapse rates showed the inverse pattern, steadily increasing as a function of number prior treatment failures (
5). In a large 2-year observational study, patients with severe TRD received treatment as usual (TAU), including medication changes across a broad range of antidepressants, off-label use of other psychotropic classes such as stimulants and mood stabilizers, augmentation strategies, and ECT (
1). The sample had not benefited from an average of 4.3 adequate antidepressant trials in the current episode. The TAU remission rates at 3, 12, and 24-months were 1.7%, 3.6%, and 7.8%, respectively (
1). The response rates were also unimpressive: 5.8, 11.6 and 18.4, at the three timepoints. Neuromodulation interventions, such as implanted vagus nerve stimulation (VNS) or deep brain stimulation (DBS) (
2,
4) are the treatments often studied in clinical trials with severe TRD samples. While such interventions may improve outcomes relative to TAU (
2), they also carry the risks associated with surgical interventions.
Psilocybin has shown a significant antidepressant effect in studies of patients with MDD (
6–
8). Two studies examined its efficacy in TRD specifically (
7,
8). In an open-label study of 12 TRD patients, the psilocybin intervention was associated with significant decreases in symptoms at 1 week and 3 months posttreatment (
7). In a recent, large, double-blind randomized controlled trial (RCT) of 233 patients with TRD, on the primary outcome of MADRS score at week 3, a single 25-mg dose of psilocybin, but not 10 mg, demonstrated significant antidepressant effects compared to the control 1-mg psilocybin dose (
8). In these studies, either all or the majority of enrolled patients had four or fewer treatment failures preceding the current episode (
7,
8). No study has assessed the antidepressant efficacy of psilocybin specifically in patients with severe TRD. Moreover, patients with psychiatric comorbidities or in a first depressive episode with duration of over 2 years were previously excluded (
8). The current trial documented antidepressant efficacy of psilocybin in severe TRD patients, with more than four failed treatments preceding the current episode, and no limit to the current episode duration. History of psychosis was exclusionary, but patients with comorbid PTSD or GAD were not excluded. Here we report, in this severe TRD patient sample, outcomes following a single dose of synthetic psilocybin combined with psychological support. Clinician-rated depression assessments were made at baseline and the primary 3-week timepoint, with additional assessments up to 12 weeks posttreatment. Secondary measures included self-rated depressive and anxiety symptom severity and quality of life indices. Exploratory measures included quality of the psychedelic experience and potential effects of comorbidity on antidepressant outcomes. As this patient population may also carry unique risks, a safety assessment of the intervention was an important objective.
Methods
Study Design and Patients
This was an open-label trial of a single dose of 25 mg of synthetic psilocybin administered to 12 patients with severely treatment-resistant depression (severe TRD), who were followed up for 12 weeks following psilocybin dosing. Psilocybin was provided in a proprietary formulation by COMPASS Pathways (COMP360). The study received institutional review board approval via the Sheppard Pratt Health System and the Food and Drug Administration. A Certificate of Confidentiality was obtained. All patients provided written informed consent prior to enrollment. The trial was registered on clinicaltrials.gov (#NCT04433858).
Eligible participants were 18–65 years of age, with a primary diagnosis of single or recurrent episode of major depressive disorder (MDD), with at least five treatment failures during the current episode. Diagnoses had been made initially by outpatient providers as verified by medical records, and all diagnoses were confirmed according to DSM-5 criteria by clinical assessment, and the Mini International Neuropsychiatric Interview (MINI version 7.0.2) (
9) by the study physician. Treatment failures could include FDA-approved medications administered at adequate dose and duration per MGH-ATRQ criteria (
10), neurostimulation (a treatment course of TMS or ECT to completion, i.e., not discontinued due to intolerability), and up to one failed trial of any form of psychotherapy (i.e., patient report of having met regularly with a psychotherapist during the current episode). Exclusionary criteria included any history of psychosis, personality disorders, or of concurrent substance use disorders, as detected by self-report, medical records, or clinical interview. Individuals with active suicidal ideation were excluded. Participants with comorbid diagnoses of generalized anxiety disorder (GAD) or posttraumatic stress disorder (PTSD) were included provided the primary diagnosis was major depressive disorder. Participants underwent normal screening bloodwork and an ECG.
After passing screening and prior to psilocybin dosing, patients were weaned over a period of 3–6 weeks from all medications prescribed for MDD. The minimum time between medication discontinuation and dosing was 2 weeks. The only psychotropic medications allowed to be continued at study start through week 3 were benzodiazepines (lorazepam, alprazolam, clonazepam) in the case of four patients who had used these medications long term.
During the run-in period, patients met for at least 3 hours with one of three lead therapists, all of whom were specially trained doctoral-level psychologists. Specialty training consisted of trauma therapy certification and an additional 60 hours of specialized psychedelic therapy training according to the COMPASS Pathways protocol. These sessions were to build therapeutic rapport, provide psychoeducation, and prepare for the psychedelic experience. The lead therapist and an assistant therapist were present for the duration of the 8–9 hour psilocybin dosing day. The dosing room was designed to provide a nonclinical and calming environment. Patients were encouraged to listen to a pre-designed playlist and wear eyeshades. Therapists provided as needed psychological support. The study physician was on-site for the dosing day and met with the patient at the end of session when psychedelic effects had dissipated to confirm safety to return home in the care of a significant other. Patients returned to the site 1 day post-dosing for assessment by the study physician and the first of three 1-hour integration sessions with the lead therapist. Subsequent integration sessions occurred 1 and 2 weeks posttreatment. Sessions were nondirective with the goal of supporting the patient in consolidating their insights from the experience. Patients’ clinical status was monitored throughout the study by regular visits with the treating psychiatrist as well as a therapist. Primary clinician rating scales were administered at baseline and 1, 2, 3, 6, 9, and 12 weeks post-dosing, performed by the study physician in a one-on-one setting with each participant. Patients were requested to remain off psychotropic medications for the first 3 weeks after dosing but to alert the study physician at any time during the study of concerning symptoms including agitation, insomnia, or mood symptoms that would warrant assessment of whether to restart medication. All medication restarts were documented.
Outcome Measures
The primary outcome was change in clinician-rated Montgomery-Åsberg Depression Rating Scale (MADRS) (
11) score from baseline to 3 weeks post-dosing. Secondary endpoints included change in MADRS scores from baseline at follow-up visits 1 week and 12 weeks post-dosing, and percent of participants meeting response (MADRS ≤50% of baseline) and remission (MADRS score ≤10) criteria at each follow-up visit.
Secondary and self-reported outcome measures included the Quick Inventory of Depression Symptoms-Self Rating-16 (QIDS-SR-16) (
12), the Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (QLES-Q-SF) (
13), and the General Anxiety Disorder-7 (GAD-7) (
14) questionnaire. These scales were administered at baseline and subsequent visits. Safety outcomes were assessed per clinician observation. Patients were clinically screened at each visit for potential worsening of depressive symptoms including suicidality. Monitoring for adverse events was done via direct observation by the lead therapists during the day of dosing, with subsequent screening and monitoring via clinical interviews by the study physician, with appropriate interventions given as needed.
For the purposes of hypothesis generation, given the inclusion of patients with PTSD, presence of PTSD comorbidity was tested for association with treatment outcomes. Other exploratory measures involved assessments of the subjective psychedelic experience via the Challenging Experience Questionnaire (CEQ) (
15), the Mystical Experience Questionnaire (MEQ) (
16), and the Five Dimension Altered State of Consciousness Scale (5D-ASC) (
17). Among the five dimensional subscales of the 5D-ASC as defined by Dittrich et al. (
17,
18), oceanic boundlessness (OB) was selected as the measure of interest in tests of association with treatment response, based on evidence in the literature that OB is a predictor of the antidepressant effect of psilocybin (
19).
Statistical Analyses
Statistical analyses were performed in SPSS v27 (IBM Corp., Armonk, NY). All analyses of treatment effect on primary (MADRS) and secondary measures involving more than two time points (MADRS, QLES, GAD-7, QIDS-SR-16) used linear mixed-effects models for repeated measures, with time as the fixed effect and participant as the random effect. Time points were coded as categorical variables since the primary interest was in the comparison of baseline versus posttreatment rather than in the longitudinal trend. Bonferroni-corrected post-hoc testing was used to compare each posttreatment timepoint with the baseline score to detect significant changes in means. For analyses of treatment effect comparing baseline measures to a single posttreatment time point (CEQ, MEQ, WSAS), paired t-tests were used. One patient withdrew prior to week 6; last observation carried forward was used for subsequent timepoints for this participant.
For exploratory analyses investigating PTSD comorbidity as a covariate, linear mixed models for repeated measures were used with the fixed effects of PTSD comorbidity, time, and PTSD-by-time interaction on the dependent variable (MADRS score) with participant as the random effect. All posttreatment weeks at which MADRS scores were collected (weeks 1, 2, 3, 6, 9, 12) were used in this analysis, as opposed to only the primary and secondary timepoints of interest (weeks 1, 3, 12), which had been a priori specified in the efficacy analyses. To explore the potentially mediating effects of subjective psychedelic experience via the OB subscale of the 5D-ASC, Pearson correlation coefficients were computed for the associations between OB versus MADRS scores. Multiple linear regressions were then used to assess effects OB, PTSD, and OB-by-comorbidity interaction on MADRS scores, at each of three outcome timepoints (weeks 1, 3, and 12). All statistical tests were two-tailed, with an alpha of 0.05.
Results
Patient Disposition and Characteristics
This study enrolled 12 patients from 205 individuals pre-screened (Figure S1 in the
online supplement). Demographic and clinical characteristics of the patient sample are given in
Table 1. There were equal numbers (N=6) of female and male patients. Mean age was 40.5 years. Five patients were found to have comorbid PTSD diagnosis in addition to severe TRD. All patients were followed from baseline assessments through week 3. One patient withdrew from the study prior to week 6, such that 11 patients were followed to the study end at week 12.
The mean number of medication failures in the current episode was 5.6. Among patients’ medication regimens at time of enrollment, weaned prior to dosing, the most common agents were norepinephrine-dopamine reuptake inhibitors (NDRI) which were part of the regimen of six patients (50%). Selective serotonin reuptake inhibitors (SSRIs) were being taken by three patients (25%) at enrollment, with an additional four patients (33%) having received an SSRI within 6 months of study start. Serotonin-norepinephrine reuptake inhibitors were being taken by two patients (16.7%) at enrollment and an additional two patients (16.7%) within 6 months of study start. Other medication classes being taken at enrollment, present in the regimens of ≤2 patients (16.7%), included tetracyclic antidepressants (TeCAs), serotonin antagonist and reuptake inhibitors (SARIs), serotonin modulator and stimulators (SMS), second-generation antipsychotics (SGAs), and mood stabilizers.
Efficacy Outcomes
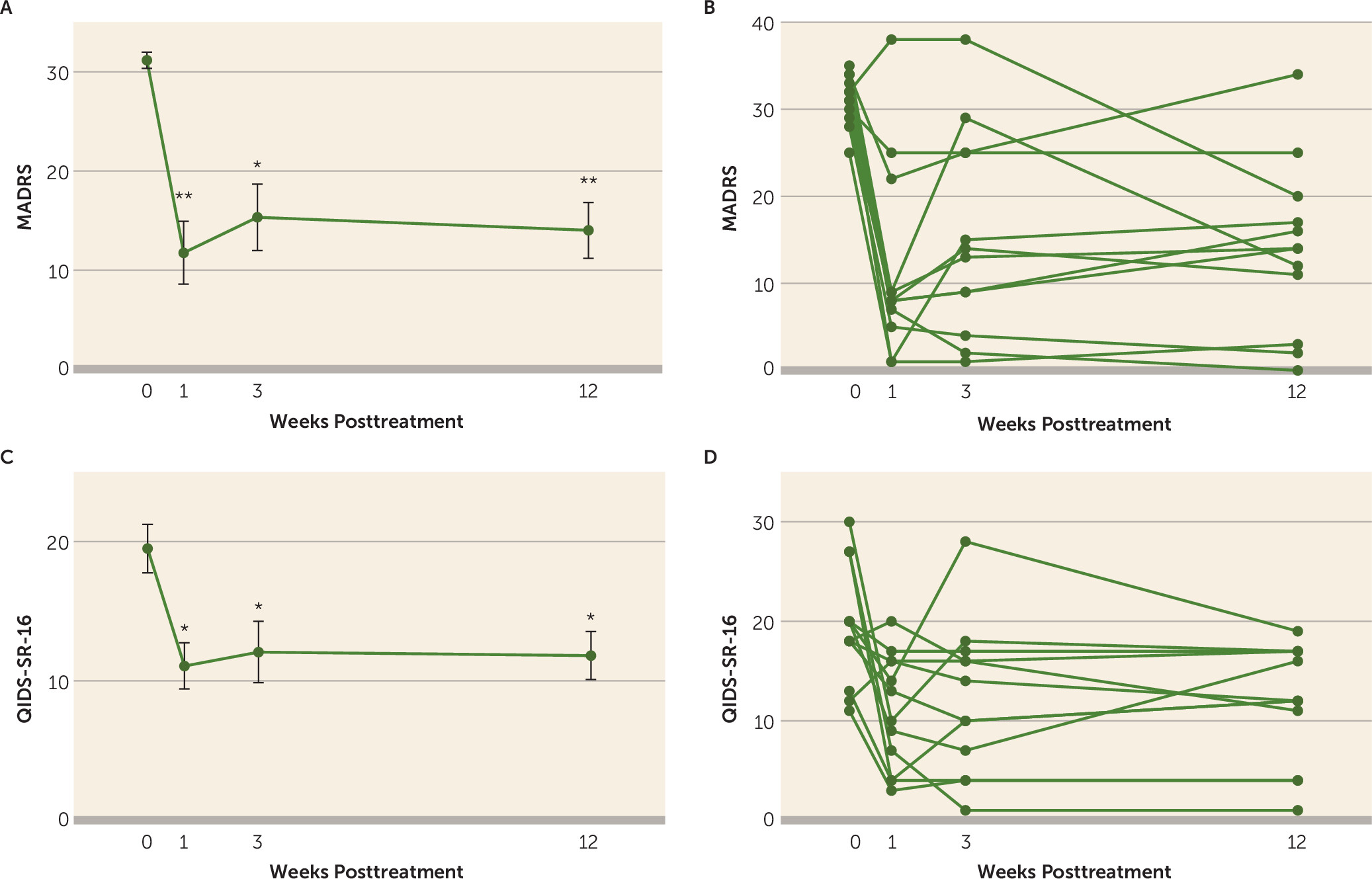
There was a significant reduction in MADRS scores from baseline at the primary and secondary endpoints (parts A and B of
Figure 1). The fixed effect of treatment (time) on MADRS scores in the overall mixed-effects model was robust (F=26.69, df-3, 25, p=6.46×10
–8). MADRS scores at each post-dosing time point were significantly reduced relative to baseline (week 1: least-squares mean change=−19.4, 95% CI=−28.5 to −10.4 [Bonferroni-adjusted p=0.0002, Cohen’s d=2.8]; week 3: least-squares mean change=−15.8, 95% CI=−25.4 to −6.268 [Bonferroni-adjusted p=0.002, Cohen’s d=2.2]; week 12: least-squares mean change in MADRS=−17.2, 95% CI=−25.2 to −9.1 [Bonferroni-adjusted p=0.0002, Cohen’s d=2.7]).
At week 1, 75% of patients met both response and remission criteria (Figure S2 in the online supplement). At Week 3 (primary endpoint), 66.7% and 41.7% of participants met response and remission criteria, respectively. Greater than 50% of patients met response criteria at all timepoints assessed with the exception of week 9. At week 9, 33% of patients met response criteria, all of whom also met remission criteria. At study endpoint (week 12) seven out of all 12 enrolled patients (58.3%) met response criteria and three patients (25%) were in remission.
The QIDS-SR-16 served as a brief secondary measure of self-rated depressive symptom severity (parts C and D of
Figure 1). The fixed effect of treatment (time) on QIDS-SR-16 scores in the overall mixed-effects model was significant (F=5.10, df=3, 21.8, p=0.008). QIDS-SR-16 scores were significantly reduced from baseline at week 1 (least-squares mean change=−8.4, 95% CI=−14.7 to −2.18 [Bonferroni-adjusted p=0.006, Cohen’s d=1.4]), week 3 (least-squares mean change=−7.4, 95% CI=−14.7 to −0.13 [Bonferroni-adjusted p=0.045, Cohen’s d=1.1]), and week 12 (least-squares mean change=−7.7, 95% CI=−14.0 to −1.3 [Bonferroni-adjusted p=0.015, Cohen’s d=1.3]).
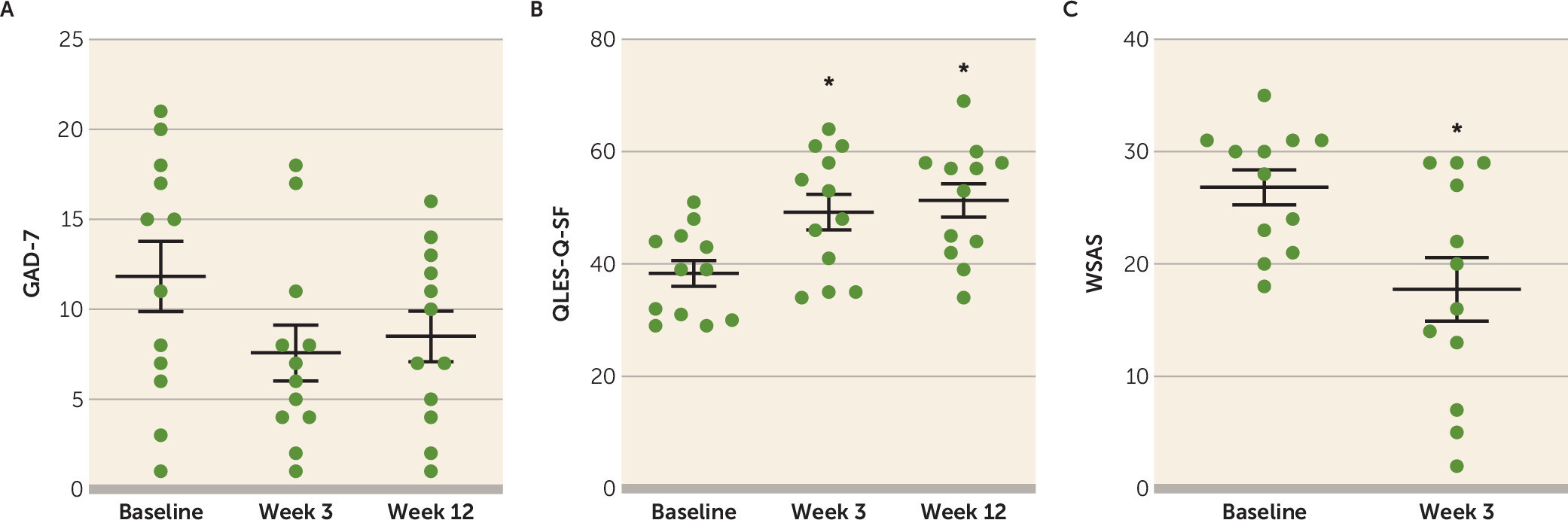
The GAD-7 provided a brief assessment of anxiety symptoms (part A of
Figure 2) and did not show a significant effect of treatment (F=1.5, df=2, 24.6, p=0.24). Quality of life, enjoyment, and satisfaction measures as per the QLES-Q-SF were compared between baseline, week 3, and week 12 (part B of
Figure 2). There was a significant effect of time in the overall model (F=7.36, df=2, 22.3, p=0.004), with significantly increased QLES-Q-SF scores from baseline at both post-dosing time points (week 3: least-squares mean change=10.9, 95% CI=1.4 to 20.4 [Bonferroni-adjusted p=0.023, Cohen’s d=1.15]; week 12: least-squares mean change=13.0, 95% CI=3.9 to 22.1 [Bonferroni-adjusted p=0.005, Cohen’s d=1.70]). The WSAS scale was used to compare impairment in work and social functioning at baseline and week 3 (part C of
Figure 2). The effect of time was significant (t=2.58, df=11, p=0.026), with the paired difference showing reduced WSAS scores at week 3 (least-squares mean change=−9.08, 95% CI=−16.8 to −1.3 [Cohen’s d=1.06]) indicating less impairment.
Safety Outcomes
There were no serious adverse events during the study (
Table 2). On the day of dosing, two patients (16.7%) reported headache, rated as mild in severity in both cases. One patient did not require medication and had resolution in 3 days. One patient took as-needed medication and had resolution in 1 day. Two patients had subacute onset of moderate insomnia, 1 week and 4.5 weeks after dosing. The former of these had additional onset of moderate psychomotor agitation 2 weeks after dosing and required medication for these symptoms, with resolution 7–8 weeks after onset. The second patient with insomnia did not require medication and experienced resolution after 2 weeks. One patient had worsening depressive symptoms with onset on the day of dosing, of moderate severity. This patient required close safety monitoring by the study physician, and restarted SSRI antidepressant at week 4, which coincided with resolution of the worsened symptoms. This patient maintained nonresponder status with respect to antidepressant effects of psilocybin throughout the study period. During the study period one patient reported self-initiating low-dose psilocybin (<3.5 g dried psilocybe mushroom, described as “microdosing”) taken irregularly over a period of 2 weeks starting at week 4. This patient was classified as a responder by MADRS criteria at study endpoint, albeit with potential confound from these additional low doses. Another patient who had used a medical cannabinoid as needed prior to the study reported resumption of its use at week 4. This patient had met remission criteria at week 3 and was also classified as remitted at study endpoint. One patient who had not achieved response at week 3 restarted an SSRI+NDRI regimen but continued to show nonresponse by MADRS criteria at study endpoint. Medication restarts are also included in
Table 2.
Exploratory Measures and Analyses
Exploratory analyses of the relationship between comorbid PTSD and antidepressant outcomes were conducted. Among the 12 enrolled patients, five had comorbid PTSD. The PTSD patients consisted of two male and three female patients (mean age=42.5 years), and the non-PTSD patients consisted of four male and three female patients (mean age=39.0 years). The comorbidity subgroups did not differ in age or sex. MADRS scores patients with and without PTSD comorbidity were compared across all timepoints (part A of
Figure 3) using linear mixed models. There was significant main effect of time (F=26.34, df= 6, 23, p=2.6×10
–9), significant main effect of PTSD comorbidity (F=25.83, df=1, 56, p=1.6×10
–6), and a significant PTSD-by-time interaction (F=3.36, df=5, 23, p=0.016). Bonferroni-corrected post-hoc comparisons between PTSD and non-PTSD subgroups at all timepoints revealed a significant difference in MADRS scores at week 6 (least squares mean difference=−11.4, 95% CI=−20.4 to −2.4 [Bonferroni-adjusted p=0.018]) and the 12-week study endpoint (least-squares mean difference=−14.4, 95% CI=−23.0 to −5.8 [Bonferroni-adjusted p=0.004]). Longitudinal MADRS scores of patients with versus without PTSD comorbidity are shown in part A of
Figure 3, and corresponding QIDS-SR-16 scores are shown in part B of
Figure 3.
The CEQ, MEQ, and the 5D-ASC assessed aspects of the psilocybin experience. The CEQ and MEQ scores on day 1 and week 3 post-dosing were compared to determine whether patients’ evaluations of the positive and negative aspects of the experience changed over time (Figure S3 in the online supplement). Measures of both challenging experience via the CEQ and mystical experience via the MEQ did not manifest significant change over these timepoints, suggesting non-diminishment of the subjective salience ascribed to the experience.
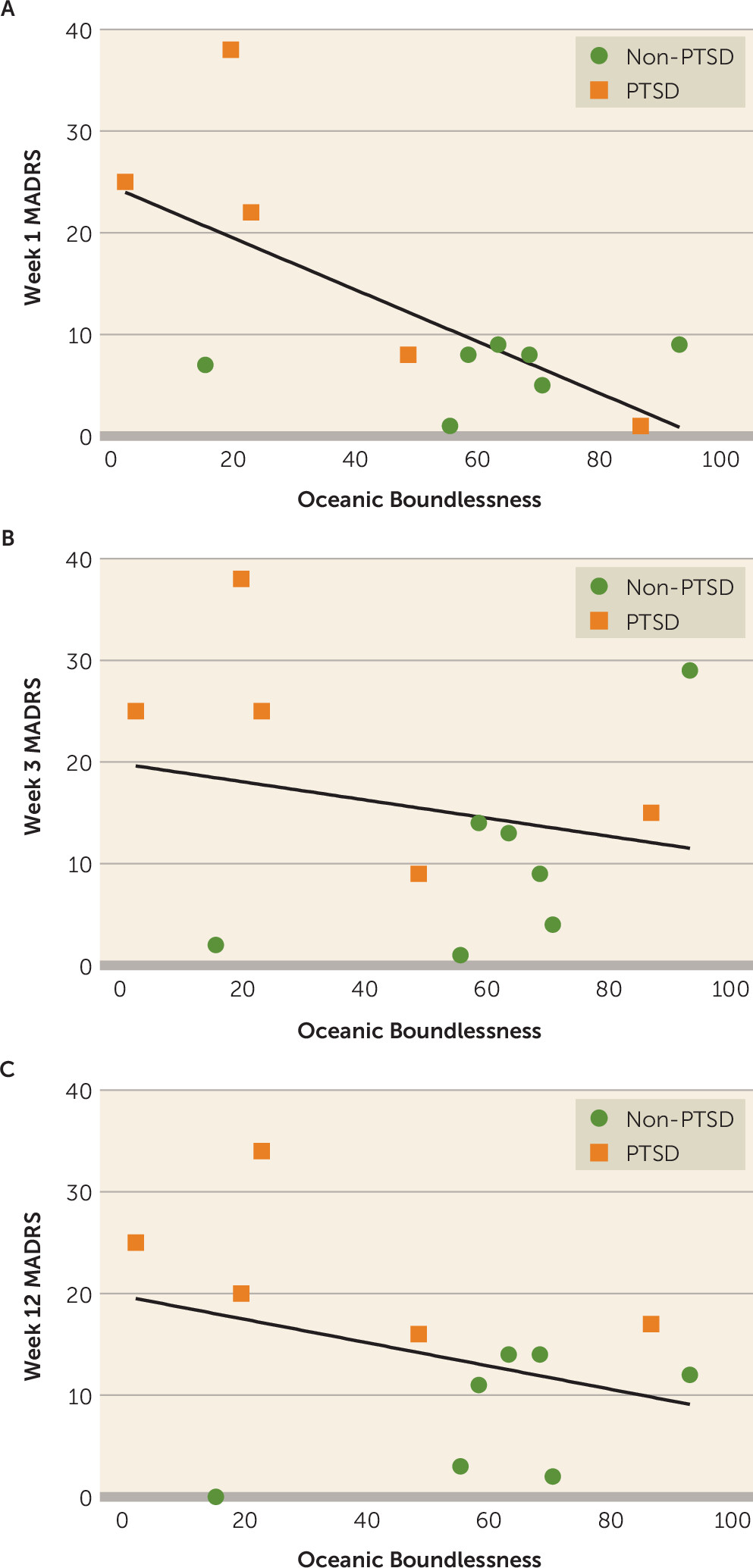
It was hypothesized that particular aspects of the psychedelic experience, as measured by the OB subscale of the 5D-ASC, covary with the magnitude of antidepressant effects following psilocybin dosing. Scatterplots representing OB versus MADRS scores are presented in
Figure 4. OB scores were significantly negatively correlated with MADRS scores at week 1 (r=−0.68, df=10, p=0.016) but not at week 3, (r=−0.22, df=10, p=0.48) or week 12 (r=−0.34, df=10, p=0.27). Multiple linear regression was subsequently applied to explore the potential interaction between OB and PTSD comorbidity on posttreatment MADRS scores. There was a significant PTSD-by-OB interaction effect at week 1 (t=−2.82, p=0.023) and week 3 (t=−2.55, p=0.034) but not at week 12 (t=−2.05, p=0.074).
Discussion
Like TRD as a whole, the phenotype of
severe TRD lacks a formal consensus defining its boundaries. It is generally recognized that treatment-resistance is not an all-or-none phenomenon but rather exists along a continuum (
20,
21). In this study, the primary inclusion criterion was failure to benefit sufficiently from at least five trials of accepted antidepressant treatments given at adequate dose and duration in the current episode, only one of which could be a form of psychotherapy. This would correspond to a score >2 on the treatment dimension of the Maudsley Staging Method for TRD (
22) and would be expected to broadly correspond to TRD Stages III–V according to the Thase and Rush Staging Method (
23).
In patients whose illness does not respond to treatment, serial antidepressant trials are known to exhibit precipitous decreases in the likelihood of remission, such that remission rates after a fourth antidepressant trial are between 10% and 15% (
5,
24–
26). In this study, remission rate at the week 3 primary endpoint was 41.7%, and at study end (week 12) 25% of patients met remission criteria. At both of these timepoints, the majority of patients manifested clinical response. In the overall sample, reductions in MADRS scores were highly significant post-dosing (part A of
Figure 1), with least-squares mean changes of −19.4, −15.8, and −17.2 at weeks 1, 3, and 12 respectively. The individual trajectories of the participants in this trial (e.g., part B of
Figure 1) are suggestive of distinct response profiles; three groups may be roughly approximated on visual inspection—corresponding to nonresponders, responders, and remitters. Additional clinical characteristics and particularly biometric data such as EEG or neuroimaging may be valuable in establishing predictors of response in future, larger studies.
One clinical factor explored here for potential interaction with treatment response was comorbid PTSD. Albeit with small sample size, exploratory analyses suggested that comorbid PTSD was associated with decreased antidepressant response. In the seven patients in this study without comorbid PTSD, all had endpoint MADRS scores less than 15, and were classified as either in response or remission. This finding, requiring replication in larger samples, may suggest that patients with severe TRD but without clinical trauma symptoms represent a subgroup that particularly favorably responds to single-dose psilocybin with psychotherapeutic support.
Safety outcomes continue to be an important consideration. There were no serious adverse outcomes in this study of psilocybin dosing in a controlled setting with substantial psychotherapeutic support. Headache and insomnia were the most common adverse effects, each occurring in two patients. The worsening depression of moderate severity in one patient highlights the importance of close clinical monitoring after psilocybin use in patients with TRD, as unmonitored use could be detrimental or dangerous in such cases. One patient reported “microdosing” psilocybe mushrooms starting in week 4, which may be considered an attempt to recreate an initial therapeutic effect that had subjectively worn off by week 3 (see patient 2 in
Table 2). This finding suggests utility for urine drug screening in clinical trials of psilocybin to assess for potential confounds from self-dosing and may warrant consideration regarding the potential risk for psychedelic usage outside of clinical settings following clinical exposure.
This study has a number of limitations. The small sample size and lack of racial diversity limit interpretability and applicability of the findings. These cautions apply particularly to the exploratory results. While the sample size itself was limited by the strict criteria to define severe TRD, larger and more diverse patient samples will be needed in future studies. During this study, two patients restarted antidepressant regimens, while one patient restarted a cannabinoid and another started microdosing psilocybin (
Table 2). The final endpoint data are subject to the confounds introduced by this medication/drug exposure. However, no patient started a new or previous compound prior to the primary endpoint (week 3).
As an initial foray into psilocybin treatment for patients with MDD that is difficult-to-treat, this study provided an early indication of safety, tolerability, and promising potential efficacy. An unexplored question concerns the durability of the antidepressant effect beyond 12 weeks and whether durability can be extended with additional dosing. Prior work in patients with severe TRD has shown remarkably low rates of response and remission with treatment as usual (
1). Future studies should include longer follow-up periods and conditional arms that examine the impact of continuation or maintenance psilocybin protocols with consideration given to a multiple dosing schema for patients with PTSD.