The Diagnostic and Statistical Manual of Mental Disorders (DSM [
1]) provides a limited set of criteria for diagnosing traumatic brain injury (TBI). According to the DSM, a diagnosis of postconcussional disorder requires: A) a history of head trauma causing cerebral concussion, B) objective evidence of difficulty in attention or memory, and C) three or more symptoms (fatigue, sleep disturbance, headache, vertigo/dizziness, irritability/aggression, anxiety, depression, affective instability, personality change, and/or apathy) occurring shortly after the trauma and lasting for at least 3 months. The symptoms in criteria B and C must follow the head trauma or represent a worsening of preexisting symptoms and must cause significant social or occupational impairment, representing a significant decline from previous functioning. A recent consensus group has more concisely defined TBI as “an alteration in brain function, or other evidence of brain pathology, caused by an external force” (
2). Alterations in brain functioning may include a period of loss of consciousness or alteration of consciousness, posttraumatic amnesia (a deficit in encoding new memories), global or specific neurologic deficits, and/or evidence of brain pathology (e.g., abnormalities detected in neuroimaging assessments). TBIs may result from external force, sudden acceleration/deceleration of the head, any forces to the head caused by blasts or explosions, and/or a foreign body penetrating the head (
2). The type, direction (e.g., angular, rotational, shear, and translational forces), intensity, and duration of forces all contribute to the characteristics and severity of TBI. Damage may occur directly under the site of impact, on the side opposite the impact (coup and contrecoup injuries, respectively [
3]), or may be diffuse.
TBI represents a serious public health problem in the United States. The Center for Disease Control and Prevention estimated that 1.7 million people sustain a TBI annually in the U.S., which leads to 52,000 deaths, 275,000 hospitalizations, and 1.4 million emergency department visits (
4). TBI is a contributing factor in 30.5% of all injury-related deaths in the United States (
4). Overall, direct and indirect medical costs from TBIs were $60 billion in the US in 2000 (
5). Of those hospitalized, 70,000 to 90,000 people per year sustain substantial injuries that will necessitate long-term or lifetime care (
6,
7). Additionally, TBI is a risk factor for other medical conditions, including depression, substance abuse, and posttraumatic stress disorder (PTSD), which entail additional costs (
8–
11). The costs are even higher when considering lost productivity and earnings for patients and their caregivers, diminished tax revenue, and the burden on social programs (
7,
11,
12). Of course, financial costs cannot capture the effects of TBI on family members and caregivers, who report increased stress and major disruptions in family functioning (
13–
15).
Approximately 75% of TBIs are concussions, also termed mild TBI (mTBI), characterized by alteration of consciousness or loss of consciousness for no more than 30 minutes (
6). Falls are the most common cause of TBI (32.5%), followed by motor vehicle injuries (17.3%), being struck against the head (16.5%), assaults (10%), and other causes (21%) (
4). Falls result in the greatest number of emergency department visits and hospitalizations, but motor vehicle injuries account for the most TBI-related deaths, especially for adults aged 20–24 (
4). Children up to 4 years of age, adolescents 15–19 years old, and adults over age 65 are most likely to sustain a TBI, although adults 75 years and older experience the highest rates of TBI-related hospitalization and death (
4). Across all age groups, TBI rates are higher for males than for females (
4). Additionally, TBI incidence rates appear to be increasing. From 2002–2006, fall-related TBIs in children younger than 14 increased 62%, and in adults over 65, there was a 46% increase in emergency department visits, 34% increase in hospitalizations, and 27% increase in TBI-related deaths (
4). Across all age groups, TBI-related emergency department visits increased by 14% and hospitalizations increased by 20% during this time period (
4).
TBIs can be classified as mild, moderate, and severe. Commonly used diagnostic guidelines include ratings of injury severity on the Glasgow Coma Scale (GCS) and criteria that include duration of posttraumatic amnesia and loss of consciousness (see
Table 1). Additionally, several scales have been used or are proposed for diagnosing and grading concussion (see
Tables 2 and
3). It has been suggested that grading the severity of TBI may be improved through wider use of neuroimaging technologies (
16). Because the vast majority of TBIs are in the mild-to-moderate range and because those with mild-to-moderate injuries are more likely to consult a psychiatrist, this review will focus on the evaluation and treatment of mild-to-moderate TBI.
What Happens During and After a TBI?
TBIs occur due to trauma involving focal and/or diffuse injuries to the brain. Focal injuries include contusions, lacerations, and intracranial hemorrhage or hematoma. Diffuse injuries tend to result from subtle stretching and tearing of brain tissue that is less observable, yet still problematic for recovery (
17). Evaluation of the TBI patient can incorporate clinical observation/interview, neuropsychological testing, and/or neuroimaging, which is showing potential to enhance evaluation of TBIs (especially more mild forms of TBI). There are now two instruments designed for immediate assessment of concussion in sports, the Sport Concussion Assessment Tool (SCAT2) (
18), designed for athletes 10 years and older, and the computerized Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) (
19) device, which is both sensitive and specific for the neurocognitive and neurobehavioral sequelae of concussion (
20). Other technologies such as magnetic resonance spectroscopy may be useful in detecting metabolic changes; diffusion tensor imaging (DTI) may help detect subtle structural damage; functional magnetic resonance imaging (fMRI) and magnetic resonance perfusion can identify changes in functional networks; and susceptibility-weighted imaging can detect microhemorrhaging (
21). Use of combined magnetoencephalography (MEG) and DTI may also detect subtle neuronal injury in mTBI not identified by traditional CT or MRI (
22).
Symptoms of TBI vary depending on the location and extent of the damage to the brain. In more severe forms of TBI, impairments observed in the postinjury evaluation generally align well with the location and severity of the injury. A person with a moderate-to-severe TBI may experience persistent headaches, vomiting or nausea, convulsions or seizures, an inability to awaken from sleep, dilation of one or both pupils, slurred speech, weakness or numbness in the extremities, loss of coordination, and/or increased confusion, restlessness, or agitation (
23). More serious brain injuries may result in stupor, coma, or a persistent vegetative state. However, even mTBIs often result in a complex set of postconcussive symptoms that may result from neurochemical changes that occur due to the injury (
24). mTBI can result in damage to neuronal and axonal cell membranes while sparing the function of cell bodies and myelin sheaths (
25). Damage to the cell membranes precipitates changes in ionic (potassium and calcium) equilibrium, resulting in release of excitatory neurotransmitters, particularly glutamate (
26). In an effort to reestablish ionic equilibrium, the cells become more active, thereby increasing glucose metabolism. Increased metabolism may in turn result in depletion of energy stores in the cells, eventually leading acidosis and edema (
24,
27). These biological changes that occur following mTBI may result in headaches, confusion, lightheadedness, dizziness, blurred vision, tinnitus, fatigue, sleep disturbance, behavioral or mood changes, and cognitive difficulties.
The most commonly reported postconcussive symptoms are sleep disturbance, fatigue, and pain. Sleep disturbances, especially insomnia (
28), following TBI are extremely common, occurring in 30%−70% of patients (
29). Additionally, a recent meta-analysis determined that some form of sleep disturbance was reported in 25%−29% of individuals following a TBI, and that TBI patients were 2–4 times more likely than individuals in the general population to experience problems with sleep maintenance, sleep efficiency (percentage of time asleep out of time in bed), nightmares, excessive sleepiness, early awakenings, and sleepwalking (
30). Fatigue also represents a major problem for many patients, occurring in 45%−73% of TBI patients and reported as the primary symptom for 43% of patients (
31). Fatigue may last for years after the injury (
32), resulting in persistent and disabling feelings of mental, physical, and psychological exhaustion, tiredness, weariness and/or lack of energy. Lastly, pain (especially headache and neck pain) is a major problem for many TBI patients (
33). A recent review found that rates of chronic pain ranged from 43% in studies of Veteran patients to 52% in civilian studies. Chronic pain may be more prevalent in patients with mTBI (75%) compared with those with moderate or severe TBI (32%) (
34).
TBIs may damage any part of the brain or multiple parts of the brain, so neuropsychological deficits following TBI can vary in both type and severity. Impairments in attention, processing speed, learning, memory, prospective memory, and executive functioning may be present (
8,
35–
41). Evidence suggests, however, that mTBI may only have short-term impact on neuropsychological functioning, which should return to baseline within three months in most cases (
42). Research in individuals with sports-related concussions (
43), for example, suggests that following mTBI, most cognitive symptoms resolve within days to three months, however, some individuals have persistent cognitive problems beyond several months (
8,
44–
46). Recent studies have highlighted poor neuropsychological outcomes even among individuals with mild-to-moderate TBI (
33). These impairments can hinder functional recovery, including cognitive readiness for work, school, and independent living (
12,
47).
Cognitive dysfunction following TBI is often exacerbated by other biological, psychological, or social characteristics of the individual. For example, previous studies have reported increased sleep disturbances among TBI patients (
48,
49) (especially among those with comorbid PTSD [
50]), which may be related to attention and memory deficits (
51). Variations in cognitive recovery rates may also be attributable to individual characteristics such as premorbid illness perception, educational background, and life stress (
9,
36,
52–
57).
What Predicts Outcome Following TBI?
Injury severity is generally the best predictor of TBI outcomes. Individuals with moderate-to-severe TBIs tend to have extended recovery periods and significant long-term impairment. Recovery may vary depending on the aspect of functioning assessed, with 65% of patients achieving preinjury functioning in basic self-care and only 40% showing full recovery in areas of cognitive competency, major activities, and leisure and recreation (
58). Length of posttraumatic amnesia appears to be the strongest predictor of functional outcomes at 6 and 12 months postinjury (
59). Other predictors of poor outcome following moderate-to-severe TBI include older age, motor deficits, impaired pupillary reactivity, history of subarachnoid hemorrhage, and lower GCS (
60,
61).
With regard to mTBI, symptoms tend to be the most severe immediately after an injury, with gradual improvement over several hours up to 90 days (
62). About 10% of individuals with mTBI have persistent postconcussive symptoms, including headache, dizziness, fatigue, insomnia, difficulty concentrating, irritability, anxiety, depression, and sensitivity to light and noise. These symptoms may lead to social and/or vocational difficulties out of proportion to the severity of the initial injury, a condition termed persistent postconcussive syndrome (
63).
In addition to injury severity, several other factors have been studied as predictors of TBI outcomes, including the timing of the TBI within the lifespan, the setting of the TBI, the type of TBI, and the presence of more than one TBI. We discuss these factors below.
Child Versus Adult TBIs
It is commonly held that, given injuries of equal severity, children will recover better than will adults due to the plasticity of the brain at younger ages (
64,
65). However, recent animal and human research is challenging this assumption. For example, studies now indicate that a developmentally immature brain may be less capable of recovering from TBI (
66). There may also be critical periods of development during which the diffuse injuries caused by TBIs will result in lost functioning only observable at later stages of brain maturation (
66). Additionally, studies have found important complications for the younger brain following TBI, including an increased propensity for apoptosis, age-dependent parameters for cerebral blood flow and metabolism, development-specific biomarkers, increased likelihood of early posttraumatic seizures, differential sensitivity to commonly used neuroactive medications, and altered neuroplasticity during recovery from injury (
67). Therefore, differences in outcomes for older adults may reflect reductions in the ability to acquire new skills rather than reduction in plasticity, per se. In younger adults, outcomes following TBI may be more dependent on multiple factors including the severity, nature, and age at time of injury, as well as environmental influences during the recovery period (
68).
Civilian Versus Military TBIs
Although TBI is common in the general population, there is increased clinical and research interest in TBI due to its high incidence in military personnel involved in recent wars. TBI has been identified as the “signature wound” of the Iraq and Afghanistan Wars, with increased rates of TBI attributed to blast-related injuries emanating from artillery, mortar, rocket shells, mines, bombs, grenades, and improvised explosive devices (
69). Blast injuries differ from injuries related to blunt-force trauma (most frequently observed in civilian populations) in several unique ways. Blast injuries are more complicated, as the head may be exposed to multiple insults during the same event. Brain injury may be primary (from exposure to the blast pressure wave), secondary (from shrapnel or projectiles from the blast), tertiary (from being thrown to the ground or against a solid object), or quaternary (from blood loss associated with bodily injuries or inhalation of toxic gases). Therefore, diagnosis and management of individuals with blast injuries may require greater understanding of factors such as the type of blast, size of the blast, distance from the blast, and postblast trauma(s).
Despite the severity and complexity of blast-related head injuries, researchers have found few differences between blast-injured and blunt-force-injured individuals with mTBI in terms of postconcussive symptoms, psychiatric symptoms, and neurocognitive performance (
70,
71). Recent research examining postmortem brains from blast-exposed Veterans found evidence for tau neuropathology that appeared identical to the tau neuropathology, neuroinflammation, and neurodegeneration observed in brains of athletes with histories of repeat concussive injury (
72). However, studies using DTI in military personnel with blast-related mTBI have found abnormalities in regions (e.g., middle cerebellar peduncles and orbitofrontal white matter) not known to be commonly injured in civilian cases of mTBI but that are predicted to be sensitive to blast injuries (
73). Taken together, these results suggest that short- and long-term impact of blast injuries is not yet fully understood.
Effect of Repeated TBIs, Even Without Loss of Consciousness
Long-term outcome resulting from exposure to multiple concussions or mTBIs has not been well-studied and is not currently well understood. Recent studies suggest that repeated mTBI can lead to an increased risk of cognitive impairment and neurodegenerative disease later in life (
74,
75). Repeated injury appears to impact structural, functional, metabolic, and behavioral responses in the brain, and the impact of repeated injuries is most severe when the second injury occurs within hours to 1 month following the first injury (
76). It is unknown whether vulnerability to the effects of a second injury is mediated by age, gender, severity and intensity of injury, length of time between injuries, and/or related to observed metabolic and neuroimaging alterations (
76). Nonetheless, it is well-documented that repeated concussions can result in a condition now termed chronic traumatic encephalopathy (CTE). CTE is believed to result in a decline in memory and cognition over time, as well as increased risk for depression, suicide, aggressiveness, Parkinsonism, and dementia (
77), and may be associated with persistent abnormalities in visual motor and motor cortex functioning (even after recovery of other neuropsychological performance) (
78). CTE has been well-studied in boxing (
79,
80), and is now being studied in other sports, especially football (
75,
81–
84). Slower recovery rates have also been observed in patients with more than one concussion (
85). These findings suggest that repetitive head injury has significant short- and long-term ramifications that require understanding of both the severity of the initial injury and the duration between injuries as probable predictors of increased morbidity in this population (
76).
Common Psychiatric Comorbidities in TBI Patients
Individuals with a history of TBI are more likely to have psychiatric disorders, including those related to mood, anxiety, and substance abuse (
86,
87). Among TBI survivors, 43% have at least one psychiatric diagnosis, compared with only 20% of those without a TBI (
86). Psychiatric comorbidities may be even more common in veterans: about 7% of recent veterans using VA healthcare have persistent postconcussive symptoms and higher utilization of healthcare services due to high rates of PTSD (comorbid in 73% of veterans with TBI), depression (comorbid in 47%), and back/neck/headache pain (comorbid in 72%) (
88). Veterans may be more susceptible to psychiatric illness following TBI due to exposure to blasts, which may selectively impact the prefrontal cortex, leading to disinhibition of regions that regulate fear and anxiety (
89). As well, inflammation caused by TBI may also lead to increased risk for psychiatric illness (
89). Psychiatric comorbidities not only complicate the course of the injury but impede both the emotional and physical recovery from TBI (
90,
91). These comorbidities can also further contribute to cognitive impairment (
8–
10).
Mood disorders are some of the most common psychiatric complications of TBI (
92,
93). Major depression is the most prevalent, affecting at least 25% of those who have experienced a TBI (
89). The risk of developing depression is not limited to the acute stages of TBI but exists even decades after the injury (
90). The prevalence of depression within the first year following TBI ranges from 33%−42%, and rises to as high as 61% within the first 7 years (
87). Those with more severe TBI are more vulnerable to depression in the first 12 months postinjury, whereas those with milder TBI show a more prolonged onset and persistent risk of incidence (
90,
94). Despite efforts to uncover a biological basis for the development of post-TBI depression, a consistent pattern of localized lesions associated with the development of depression remains elusive (
95). In addition to major depression, those who suffer a TBI are also more likely to suffer from dysthymia and bipolar disorder (
96).
Anxiety disorders after TBI also occur at a much greater frequency than in the general population, with an overall prevalence of 29% (
56). Of the range of anxiety disorders associated with TBI, PTSD is very common (
97), with a large increase relative to the general population (
90,
98). PTSD is arguably the most expected of psychiatric sequelae, given the sometimes violent and life-threatening nature of TBI. Prevalence estimates of PTSD are highly variable, ranging from 1%−50% across studies (
96,
99,
100). Much of this variability is probably due to differences in diagnostic criteria, populations, and time postinjury. Military service members seem to be particularly susceptible to PTSD in the context of TBI (
101). Among veterans, those who screen positive for TBI are three times more likely to be diagnosed with PTSD (
102). Comorbid substance abuse and depression appear to increase vulnerability to PTSD following TBI (
103). Longer loss of consciousness and posttraumatic amnesia appear to decrease vulnerability to PTSD due decreased memory for the traumatic event, explaining why comorbid PTSD is more common in those who experience milder brain injuries (
104–
106, but also see
107). In addition to PTSD, there are increased rates of generalized anxiety disorder, panic disorder, and OCD in TBI patients (
86,
90).
Substance abuse and dependence rates are elevated for those who experience TBI, with incidence rates ranging from 28%−32% (
91,
108). Those who suffer a TBI tend to have higher preinjury substance use and abuse (
109,
110). Comorbid substance abuse and TBI is even more prevalent in the military population; in a study of veterans, those who screened positive for a TBI were twice as likely to have a substance-related disorder (
102). Substance abuse after TBI hampers recovery, increases risk of other psychiatric disorders, and increases rates of reinjury (
111,
112). Poor medical, neurobehavioral, vocational, and quality of life outcomes have also been linked with postinjury substance abuse (
110). Evidence suggests that some TBI patients decrease their drug and alcohol abuse in the short term following TBI, but this abstinence may be short-lived, with substance abuse increasing in the years following TBI (
110,
112).
Treatments
Pharmacological
There is an undoubted need to treat the cognitive, behavioral, and psychiatric symptoms following TBI. However, most comprehensive reviews in this field have concluded that pharmacological treatment of TBI does not produce substantial clinical benefit (
113). The published literature offers inadequate evidence to refute or support pharmacological treatment or to offer comprehensive standards or guidelines of such treatment following TBI (
114). Despite this, there is limited evidence (see
Table 4) suggesting that certain medications may be helpful in treating TBI sequelae and supporting recovery (
114,
115). Treatment of comorbid psychiatric conditions according to practice guidelines is recommended, but it should also be noted that TBI patients may be more susceptible to adverse effects of many drugs, making it especially important to monitor for interactions and toxicity (
116,
117). It has also been recommended that TBI patients avoid medications that lower seizure thresholds, cause confusion, or contribute to cognitive slowing or fatigue (
117).
Nonpharmacological
Nonpharmacological interventions for TBI are usually focused on symptom management, recovery of function, and community reintegration into the least restrictive living, educational, vocational, and social settings. Cognitive and postconcussive symptoms are likely to require different approaches, and there is consensus that treatment of mTBI should involve psychoeducation regarding TBI, emphasizing the expectation of full recovery (
9).
Cognitive rehabilitation.
Cognitive rehabilitation has been used in TBI, psychiatric disorders (
118,
119), and memory disorders such as Alzheimer’s disease (
120), and is generally classified as restorative or compensatory (
121). Restorative treatments use drills and practice, either paper and pencil or computer-assisted, to restore cognitive abilities, whereas compensatory interventions focus on teaching strategies to work around cognitive impairments and using spared cognitive functions to develop alternative ways to carry out activities of daily living. Compensatory strategies can be internal to the person (e.g., using visualization to remember to carry out a task) or external (e.g., using calendars, alarms, or smart devices). Interventions may also be combined with environmental approaches, which decrease the cognitive demands of the environment (e.g., simplifying a work space). Most of the extant research on cognitive rehabilitation for TBI has investigated interventions for individuals with strokes or severe brain injuries (
101,
121–
123), so little is known about rehabilitation for those with mild-to-moderate TBI (
124), especially those with chronic symptoms—the very population most likely to come to the attention of mental health professionals. Less than a handful of randomized controlled trials of cognitive rehabilitation for individuals with mild-to-moderate TBI have been conducted (
125,
126), and systematic reviews have had to rely on small, poorly designed, or uncontrolled trials (
124,
127,
128). Due to the limited research available, the VA/Department of Defense Clinical Practice Guideline for Management of Concussion/Mild TBI (
117) did not make recommendations in favor of or against cognitive rehabilitation but did recommend psychoeducation and family education regarding TBI and interventions to promote return to work and community reintegration (
117). The literature on cognitive rehabilitation in severe mental illness has demonstrated that cognitive interventions have larger effects when delivered in the context of a broader psychiatric/psychosocial rehabilitation program (
119). Along these lines, compensatory strategies have been combined with supported employment for individuals with TBI (
129).
VA researchers are currently evaluating interventions such as Cognitive Symptom Management and Rehabilitation Therapy (CogSMART) and Compensatory Cognitive Training (CCT) for veterans with mild-to-moderate TBIs. CogSMART is a 12-week, multimodal compensatory cognitive training intervention emphasizing habit learning and compensatory strategies in prospective memory, attention, learning/memory, and executive functioning. Compensatory strategies appear to be useful regardless of the etiology of the impairment (e.g., TBI, sleep disturbance, comorbid PTSD/depression). There are also modules on psychoeducation regarding TBI and strategies to improve postconcussive symptoms (sleep disturbance, fatigue, headaches, and tension). Borrowing from Mittenberg and colleagues’ seminal work on psychoeducation (
9), the intervention sets the expectation of full recovery from postconcussive symptoms. The treatment manual was developed in consultation with the Acquired Brain Injury program at Mesa College in San Diego and other cognitive rehabilitation researchers. Similar strategies had positive effects on cognition, psychiatric symptoms, functional capacity, and quality of life in a schizophrenia sample (
130). CCT is a 10-week intervention combining elements of CogSMART with Cognitive Strategy Training, which had been developed in parallel (
131). CCT is currently being evaluated in a randomized controlled trial, as is SMART-CPT, a combination of Cognitive Processing Therapy for PTSD and CogSMART strategies, for individuals with both PTSD and TBI.
Supported employment.
Rates of return to work following TBI range from 12%−70%, mainly depending on injury severity, emotional and neuropsychological functioning, self-awareness, and community support (
12,
52,
129,
132,
133). Even in mTBI patients, return to previous level of employment is not guaranteed. One study of motor vehicle accident survivors with mTBI showed that 42% of the patients returned to work, and only 12% regained employment at their premorbid level (
134). Those who return to work tend to earn less than they did prior to the TBI (
7,
135). The consequences of unemployment in this population are not merely financial but also include social isolation, low self-esteem, and increased substance abuse (
12).
Supported employment is the evidence-based practice to assist people with TBI in returning to work (
12,
129,
132) and is cost-effective (
135). Supported employment is an individualized approach to work rehabilitation based on the client’s interests and abilities and emphasizing rapid job searching for competitive work in the community (
129,
132). Employment specialists develop relationships with local employers and provide clients and employers with follow-up support as long as necessary after job placement (
133,
136). Studies show that return to work depends more on early intervention than initial injury severity (
136), indicating that successful outcomes are possible across the TBI severity spectrum.
Controversies
Several controversies regarding TBI are noteworthy. Some researchers have suggested that the term “mTBI” is over-used and favor the term “concussion” in order to help set the expectation for recovery and avoid making the event sound more serious than it is (
137). Although some have used the label “post-concussive syndrome,” others prefer “post-concussive symptoms.” There is no evidence that the symptoms typically reported following mTBI represent a syndrome, as they do not cluster in any reliable manner, nor does resolution of any particular symptom predict resolution of other symptoms (
138). Additionally, postconcussive symptoms typically vary in duration and often require separate treatments to be adequately addressed. Moreover, there is little consensus on the diagnostic criteria for concussion, with multiple scales having been published in recent decades (see
Tables 2 and
3).
The majority of those who experience mTBI will fully recover within 3 months, but a significant minority report continued postconcussive symptoms (
139), with symptoms reported to last as long as 15 years after mTBI (
8,
140,
141). There is much disagreement as to whether these persistent symptoms are due to psychiatric cormorbidies or the TBI itself (
140–
146). Recent studies have found that most postconcussive symptoms (except headache) can best be attributed to psychiatric illness (e.g., PTSD, MDD), especially in military personnel (
145). Evidence shows that anxiety, notably PTSD, mediates the relationship between TBI history and subjective measures of health and psychosocial functioning (
141,
143). It has also been demonstrated that PTSD and depression are more reliable predictors of emotional and cognitive symptoms than is TBI itself (
140,
141). Conversely, other investigations have found that comorbid conditions do not mediate this relationship entirely. After controlling for other psychiatric conditions, mTBI may still be significantly associated with headaches, sleep problems, and memory difficulties, even in the chronic phase years after injury (
140). This disagreement in the literature has led some to conclude that pathology and psychology may interact to influence the generation, reinforcement, and perpetuation of symptoms following TBI (
90). It has proven difficult to disentangle the TBI from its comorbid psychiatric conditions, suggesting that each of these conditions has its own additive properties, with each disorder serving to hinder recovery from the other (
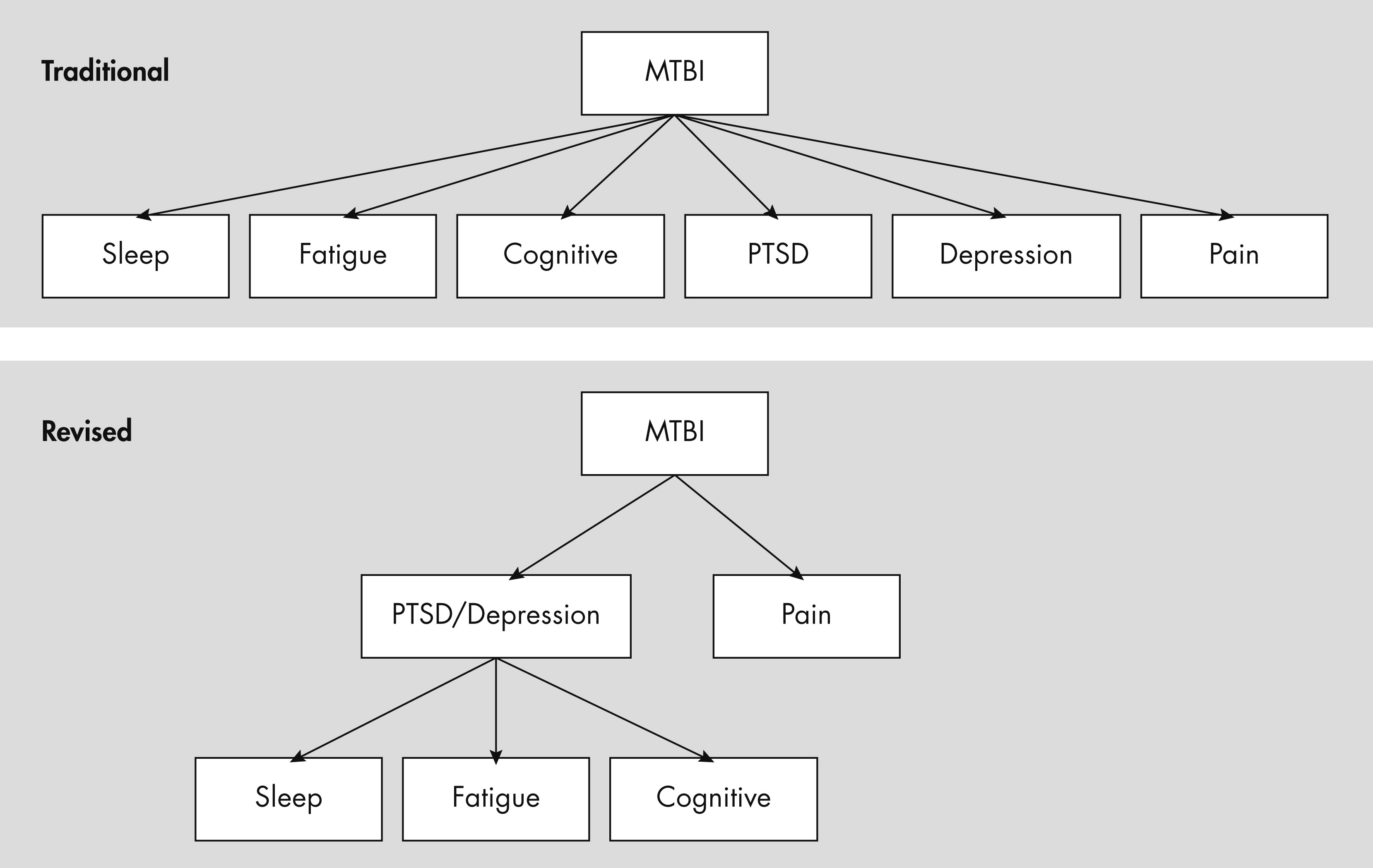
142,
147; see also
Figure 1).
The validity of neuropsychological testing following TBI is another topic of vigorous discussion. In light of multiple potential sources of secondary gain (e.g., litigation, disability benefits) measures of test-taking effort are included in neuropsychological evaluations to ensure the validity of test results (
148). Effort test scores signifying suboptimal effort do not necessarily equate to malingering, and may reflect other factors, such as fatigue, apathy, pain, psychiatric symptoms, substance use, or conversion disorder. Malingering is the intentional exaggeration or fabrication of deficits in the context of external incentives; intentionality may be difficult to prove because it is not an observable behavior (
148,
149), and the presence of external incentives is not always known. Delis and Wetter developed the term “cogniform disorder” to explain the unintentional exaggeration of cognitive symptoms (
149). In contrast to research demonstrating that postconcussive symptoms and cognitive performance normalize within the first 3 months following mTBI (
8,
45–
47), evidence suggests that individuals in litigation tend to report continued postconcussive symptoms and cognitive difficulties over time (
41). It is estimated that 40% of concussion cases involving litigation are associated with invalid test results, reflecting a range of possible explanations from unconscious symptom exaggeration to outright malingering (
32). One study (
150) found that after controlling for suboptimal effort, the cognitive performance of veterans with mTBI was indistinguishable from those who had never sustained a TBI, validating previous research that effort can account for a significant portion of the variance seen in neuropsychological testing (
148,
150).
Future Directions
Development of biomarkers for mTBI are urgently needed, and such biomarkers will be invaluable for assisting with assessment of events involving unwitnessed or unclear loss of consciousness, posttraumatic amnesia, and GCS. Biomarkers of mTBI would also be useful as outcome measures as well as predictors of outcome. With regard to treatment, despite the imprecise etiology of the cognitive and postconcussive symptoms following mTBI, it is clear that many patients presenting with these symptoms require treatment and rehabilitation to achieve their optimal functioning. Behavioral treatment modalities may be similar to those used for individuals with more severe injuries. Future research is likely to examine the effects of combination treatments, for example, the combination of restorative cognitive rehabilitation (the “bottom-up” approach that trains basic cognitive skills) and compensatory cognitive approaches (those that focus on “top-down” training of more complex cognitive and functional skills). Another combination of interest is that of pharmacological treatment with cognitive rehabilitation. Drugs that enhance specific cognitive domains (e.g., attention, working memory, learning), particularly if those cognitive domains are subserved by healthy neural circuitry, might synergistically enhance the benefits of cognitive rehabilitation. Research on combining pharmacologic and cognitive rehabilitation approaches would be further enhanced by establishing biomarkers that predict sensitivity to medication or training effects and can be used as an index of treatment outcome. Finally, taking a cue from the literature on cognitive rehabilitation in severe mental illness, we expect that cognitive rehabilitation interventions for TBI will increasingly be studied and delivered in a broader rehabilitation context that emphasizes school/work functioning and community reintegration.