The Role of Behavioral Interventions in Buprenorphine Maintenance Treatment: A Review
Abstract
Objective:
Method:
Results:
Conclusions:
Trials Showing No Benefit of Adding Counseling to Buprenorphine Plus Medical Management
Buprenorphine Treatment and the Role of Behavioral Interventions: Six Questions
Is Buprenorphine That Effective?
Is Medical Management That Effective?
Are Behavioral Interventions Ineffective in this Population?
| Study | N | Conditions | Exclusionsb | Length of Trial | Frequency of Medical Management | Behavioral Therapy | Frequency of Urine Monitoring | 6-Month Retention Ratec | Opioid-Free Urine Specimens |
|---|---|---|---|---|---|---|---|---|---|
| Fiellin et al. 2006 (5) | 166 | 3 | No alcohol, benzodiazepine, or sedative dependence | 24 weeks | 1–3 times a week | Standard medical management, 20 minutes; enhanced management, 45 minutes | Once a week | MM once a week, 50%; MM three times a week, 41%; EMM three times a week, 44% (completion confounded with attendance at counseling) | MM once a week, 44%; MM three times a week, 40%; EMM three times a week, 40% |
| Weiss et al. 2011 (3) | 653 | 2 | No heroin injection or history of heroin dependence or pain event within 6 months; no alcohol or other drug dependence requiring immediate medical attention | 24 weeks (phase 1: 4-week taper, 8-week follow-up; phase 2: 12-week maintenance + 4-week taper + 8-week follow-up) | Phase 1: twice in week 1, then once each in weeks 2, 3, 4, 6, 8. Phase 2: twice a week in week 1, then once a week in weeks 2–16 | Individual manualized drug counseling. Phase 1: twice a week in weeks 1–4, every 2 weeks in weeks 5–8. Phase 2: twice a week in weeks 1–6, once a week in weeks 7–12, no visits in weeks 13–16 | Once a week in weeks 1–16, twice a month in weeks 17–24 | Successful outcome: composite of retention and abstinence. End of phase 1: MM, 7%; MM+ODC, 6%. End of phase 2: MM, 47%; MM+ODC, 52% | Not reported by group; 61% during weeks 1–12; 42% during weeks 13–24 |
| Ling et al. 2013 (2) | 202 | 4 | No alcohol or other drug dependence requiring immediate medical attention | 32 weeks (phase 1: 16 weeks of behavioral treatment; phase 2: 16 weeks with medication maintenance only) | Twice a week for 16 weeks; checklist used | CM twice a week; CBT once a week in weeks 1–16; no behavioral therapy in weeks 17–32 | Twice a week in weeks 1–16, once a week in weeks 17–24 | CBT, 49%; CM, 57%; CBT+CM, 49%; NT, 43% (32-week retention) | CBT, 54%; CM, 46%; CBT+CM, 42%; NT, 52% |
| Fiellin et al. 2013 (4) | 141 | 2 | No alcohol, cocaine, or benzodiazepine dependence | 24 weeks (12 weeks with CBT, 12 weeks no CBT) | Once a week for the first 2 weeks, then twice a month | Clinician-delivered CBT once a week | Once a week | MM, 45%; MM+CBT, 38%. Transfer to methadone: MM, 39%; MM+CBT, 27% | Not reported |
| Bickel et al. 2008 (38) | 135 | 3 | None | 23 weeks | Not described | Standard counseling: once a week; computer CRA: three times a week; clinician CRA: three times a week | Three times a week | Standard counseling, 58%; computer CRA, 62%; clinician CRA, 53% | Standard counseling, 57%; computer CRA, 70%; clinician CRA, 73% |
| Christensen et al. 2014 (39) | 170 | 2 | None | 12 weeks | Not described | Computer CRA three times a week; all received CRA three times a week and 30 minutes of counseling every 2 weeks | Three times a week | CRA+CM, 80%; CM only, 64% (12-week retention) | Not reported |
| Schottenfeld et al. 2005 (40) | 162 | 4 | No alcohol or sedative dependence | 24 weeks; contingencies change at week 12 | Not described | Counseling: twice a week in weeks 1–12, once a week in weeks 13–24 | Three times a week | MMT+CM, 60%; MMT+PF, 75%; BUP+CM, 45%; BUP+PF, 22% | MMT+CM, 55%; MMT+PF, 50%; BUP+CM, 37%; BUP+PF, 28% |
| Miotto et al. 2012 (41) | 94 | 3 | No benzodiazepine or other substance dependence | 20-week assessment; 52 weeks total | Flexible | OTP: once a week in weeks 1–6, once a month in weeks 7–52. Primary care: once a week in weeks 1–6, once a month in weeks 7–52. Matrix Model: weekly groups | Once a week in weeks 1–9, once a month through week 52 | 20-week retention. OTP, 21%; primary care, 33%; Matrix Model, 52% | At week 20: OTP, 22%; primary care, 17%; Matrix Model, 33% |
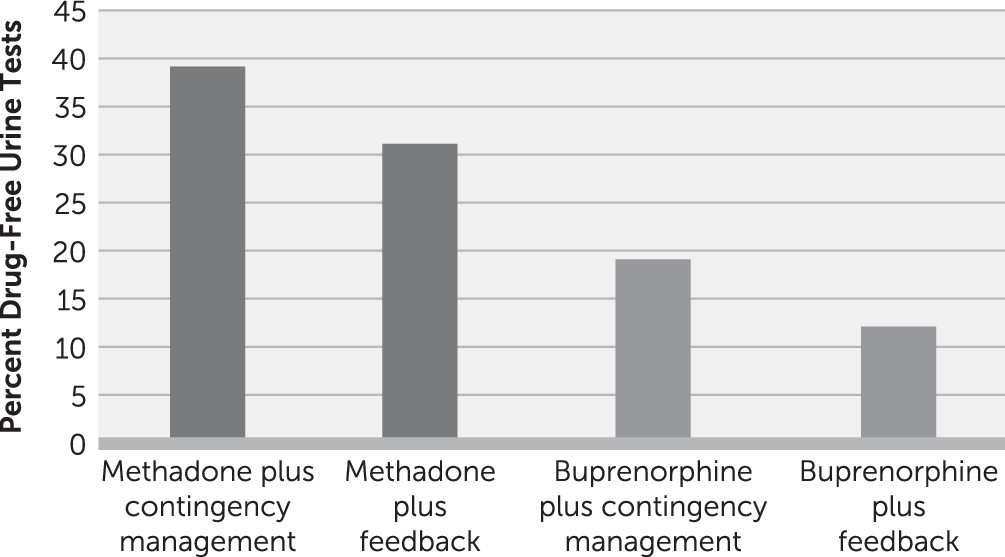
Has Research Design Affected the Results of Studies of Buprenorphine and Behavioral Treatment?
What Do We Know About Subgroups of Patients Who Do and Do Not Benefit From Behavioral Interventions?
What Outcomes Should Clinicians Aim for in Buprenorphine Maintenance Treatment?
Summary and Conclusions
References
Information & Authors
Information
Published In
History
Keywords
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

