Introduction
Depression is the leading cause of disability worldwide (
1). The number of people living with depression increased by around 18% between 2005 and 2015, and depression affects 322 million people, or about 4% of the world’s population (
1). Pharmacotherapy and psychotherapy are the two mainstays of depression treatment. In particular, second-generation antidepressants, including selective serotonin reuptake inhibitors (SSRIs), are the first-line options in the pharmacological management of major depression (
2).
However, there is still uncertainty about the dose dependency and optimal target dose of second-generation agents. Current practice guidelines provide conflicting recommendations: the National Institute of Health and Care Excellence guideline in UK states that no dose dependency has been established within the therapeutic range of SSRIs (
3), whereas the American Psychiatric Association (APA) guideline recommends titration up to the maximum tolerated dose: “Initial doses should be incrementally raised as tolerated until a therapeutic dose is reached...doses of antidepressant medications should be maximized, side effects permitting” (
4).
Systematic and comprehensive reviews of the literature examining dose dependency of antidepressants should clarify the issue and inform the guideline recommendations. Unfortunately, the available reviews are few and their conclusions disagree (
5–
7). Moreover, they addressed mainly dose-efficacy relationships and gave little attention to the balance between efficacy, tolerability, and overall acceptability of treatment.
We therefore did a dose-response meta-analysis of fixed-dose studies of commonly prescribed antidepressants for the treatment of adults with major depression (
2), examining not only their efficacy, but also their tolerability and acceptability, to provide summative evidence to inform future guideline recommendations.
Results
We identified 24 524 published records through electronic search, manual search, or personal communication, and 4030 unpublished records through industry and regulatory agency websites, contact with authors, and trial registries. 561 published and 121 unpublished full-text records were assessed for eligibility, and we included 77 studies examining various fixed doses of the included drugs: 27 studies based on published articles only, 21 studies based on unpublished records only, and 29 based on both published and unpublished records (
figure 1). The 77 studies included 201 treatment groups: placebo (75 treatment groups), citalopram (17 treatment groups), escitalopram (16 treatment groups), fluoxetine (27 treatment groups), mirtazapine (11 treatment groups), paroxetine (28 treatment groups), sertraline (11 treatment groups), and venlafaxine (16 treatment groups). The study year ranged between 1986 and 2013. The full references and the characteristics of the included studies are presented in the appendix.
The 77 studies included 19 364 participants (6881 were allocated to placebo and 12 483 to active drug). Their mean age was 42·5 years (SD 11·0), and 7156 (60·9%) of 11 749 participants for whom gender was reported were women. The median length of trial was 8 weeks (IQR 6–8). Nine studies had five treatment groups, 29 had four treatment groups, 30 had three treatment groups, and nine had two treatment groups. 40 (52%) were done in North America, 13 (17%) in Europe, and ten (13%) were cross-continental. 18 (23%) took place in secondary or tertiary care, four (5%) in primary care, six (78%) in both primary and secondary care, and 48 (62%) studies did not specify their study settings. The results of the risk of bias assessment are provided in the appendix. Many domains were rated as unclear and the overall risk of bias was rated as low in 21 (27%) studies, moderate in 55 (71%), and high in one (1%).
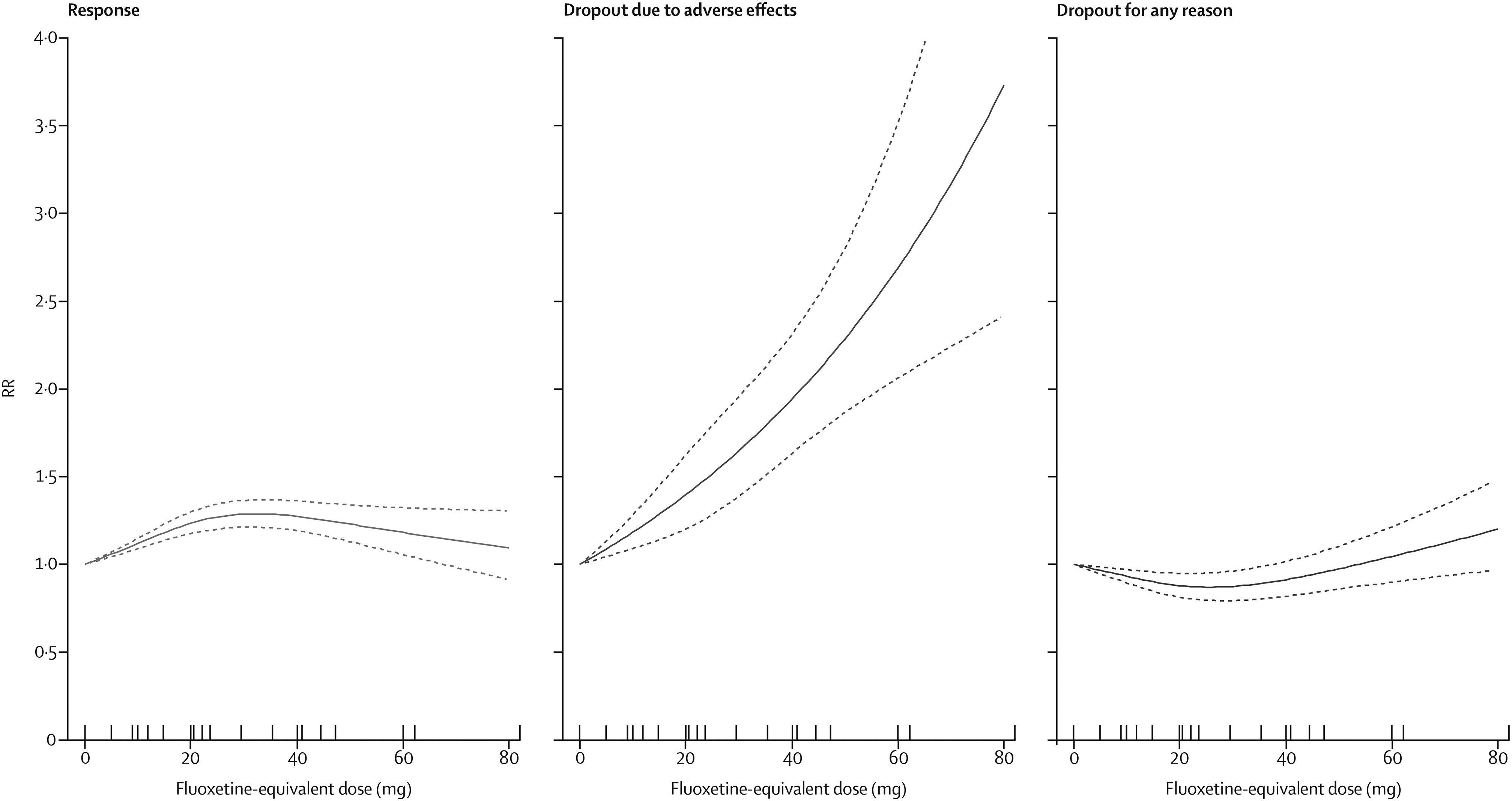
The dose-outcome relationship for treatment response, dropout due to adverse effects, and dropout for any reason for all SSRIs after the dose equivalence conversion are presented in
figure 2. The RR for efficacy gradually increased from 1·0 for placebo, to 1·24 (95% CI 1·18–1·30) for 20 mg, 1·27 (1·19–1·36) for 40 mg, and then showed a flat to decreasing trend through the higher doses (ie, 41–80 mg). Above 50 mg only a few doses were examined, resulting in less precise estimates with wider CIs in the upper dose range. The association between the dose and the dropouts due to adverse effects was linear to exponential, increasing from 1·0 for placebo, to 1·94 (95% CI 1·63–2·31) at 40 mg, and to 3·73 (2·42–5·76) at the upper limit of the licensed range (80 mg). The association between the dose and the dropouts for any reason, reflecting dropouts for lack of efficacy and low tolerability, indicates optimal acceptability in the lower range between 20 mg and 40 mg. The point estimates and their 95% CIs of RRs and risk differences (RDs) for response, dropouts due to adverse effects, and dropouts for any reason at 10–80 mg of fluoxetine equivalents of SSRIs are presented in
table 2.
The relationships between the dose and the three outcomes for each SSRI separately are presented in the appendix. The dose-outcome curves and their 95% CIs substantially overlapped, suggesting little heterogeneity among the SSRIs in their dose-outcome relationships.
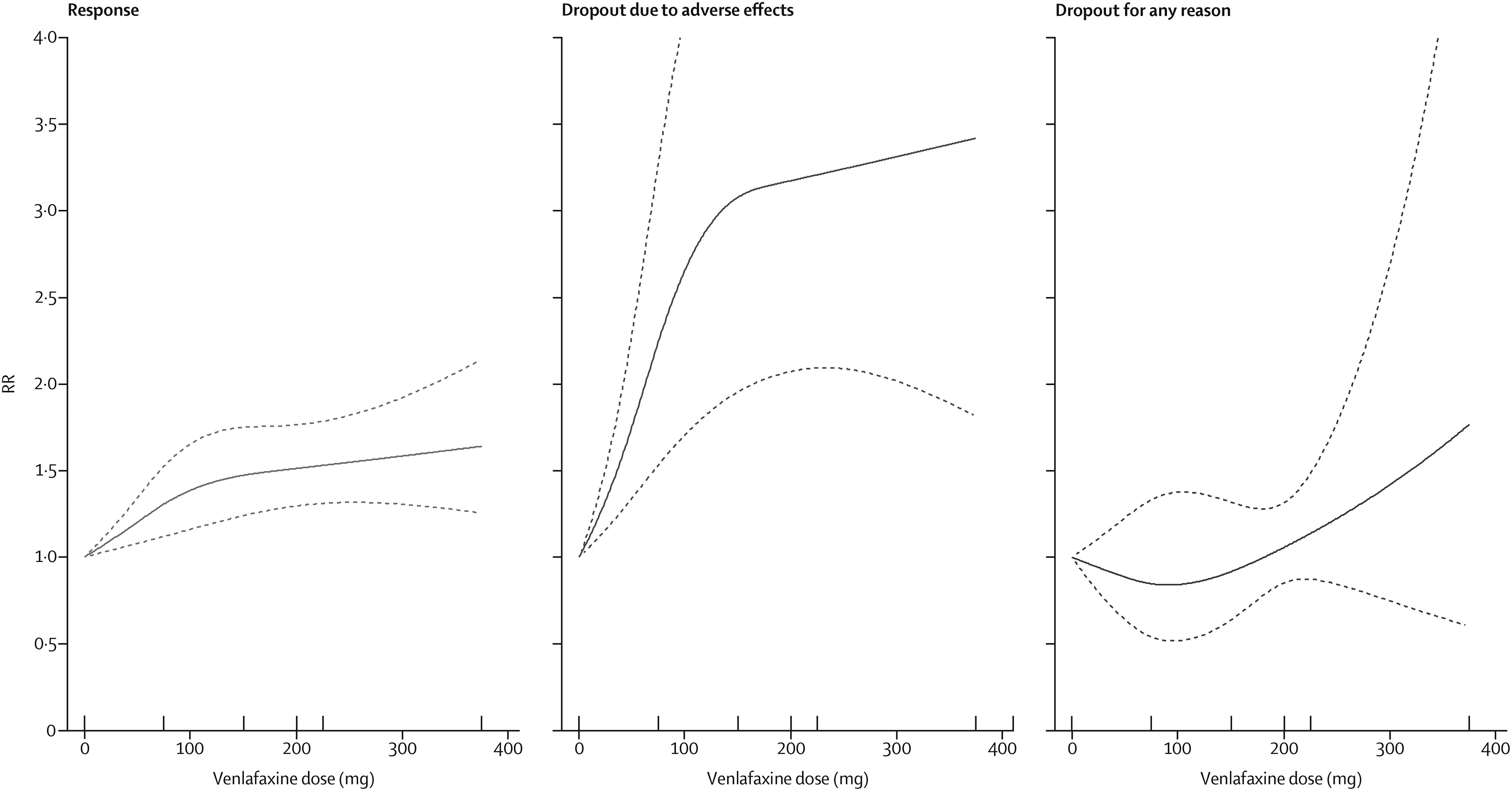
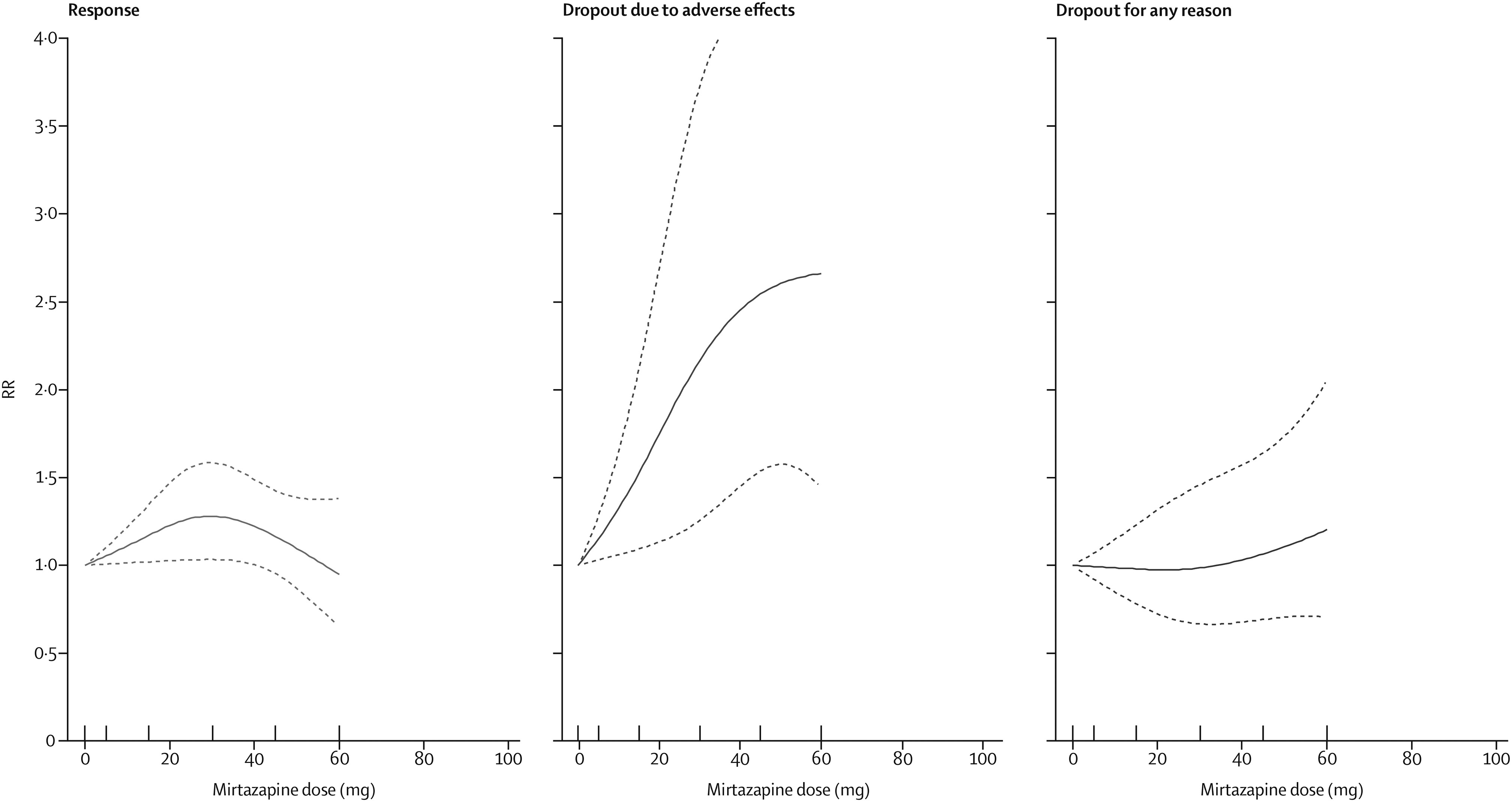
The dose-outcome relationships for venlafaxine and mirtazapine are presented in
figures 3 and
4, respectively. The efficacy of venlafaxine increased fairly steeply up to around 75–150 mg and more modestly with higher doses (ie, 151–375 mg), whereas the efficacy of mirtazapine increased up to a dose of 30 mg and then decreased. Dropouts due to adverse effects increased steeply with increasing doses for both drugs, resulting in a dose-acceptability curve that was convex at the lower licensed range, which approximately corresponded with 20–40 mg of fluoxetine equivalents in both cases. However, studies for each individual drug were few, and the 95% CIs of the spline curves remained wide.
We examined various knots when drawing spline curves for the dose-outcome curves for SSRIs (appendix). All curves overlapped with our primary analyses. When we examined different dose-equivalence calculations among SSRIs or limited to low risk of bias studies (appendix), all the results were similar to the primary results (
figure 1). The dose-remission relationship showed the same tendencies as the dose-response for SSRIs, venlafaxine, and mirtazapine (appendix).
Discussion
We did a comprehensive dose-response meta-analysis of the most commonly prescribed antidepressants, based on the largest pool of antidepressant trials for major depression (
2). For SSRIs, the probability of response increased up to doses between 20 mg and 40 mg of fluoxetine equivalents, with no further increase or even a slight decrease at higher doses within the licensed dose range up to 80 mg. Dropouts due to adverse effects showed a steep, linear to exponential, increase with increase in dose. Consequently, dropouts from all causes, including for lack of efficacy and for adverse effects, were lowest between 20 mg and 40 mg. Venlafaxine and mirtazapine showed slightly different dose-efficacy relationships; however, all drugs showed optimal acceptability towards the lower end of their licensed dose range.
These results are in line with psychopharmacological investigations. Using PET scans, Meyer and colleagues (
22) showed that approximately 80% serotonin transporter occupancy occurs at minimum therapeutic doses of citalopram, fluoxetine, paroxetine, sertraline, or venlafaxine, and that further increments in dose resulted in only small increases in transporter occupancy: a hyperbolic relationship typical of ligand-receptor binding (
23). Our data also suggest that increases in transporter occupancy above 80% do not result in greater treatment efficacy. Similarly, conventional antipsychotic drugs require dopamine D
2 receptor occupancy of about 70% to achieve a therapeutic effect; greater D
2 occupancy does not increase efficacy, but raises the incidence of side-effects (
24). Venlafaxine is a serotonin and noradrenaline reuptake inhibitor; however, functional noradrenaline reuptake blockade might become apparent only at higher doses (eg, 225 mg and 375 mg per day of venlafaxine) (
25). This dual action might be responsible for the observed increase in efficacy with higher doses for venlafaxine. Mirtazapine has more complex psychopharmacological properties, and the precise mechanisms underpinning its antidepressant action are less well understood. Mirtazapine is thought to increase noradrenaline and serotonin release through antagonism of central α2-adrenergic autoreceptors and heteroreceptors. It also exhibits antagonism to some serotonin (5-HT) receptor subtypes (5-HT
2A, 5-HT
2C, and 5-HT
3), while overall increasing tonic activation of post-synaptic 5-HT
1A receptors (
26).
Earlier reviews examining the clinical dose-efficacy relationship of antidepressants have produced variable results; some of this confusion might be due to arbitrary categorisation of doses and their inconsistent naming. Based on 33 studies of new-generation and older-generation antidepressants (n=5844), Bollini and colleagues (
5) used four categories for dose (<20 mg, 21–40 mg, 41–50 mg, and >50 mg of fluoxetine equivalents) and concluded that the response rate showed a gradual increase up to 21–40 mg, with no further increase for the two high-dose categories. The inclusion of older tricyclic antidepressants limits applicability of their review to modern practices. Hieronymus and colleagues (
6) analysed individual patient data from 11 trials of citalopram, paroxetine, and sertraline (n=2859) and found that “doses below or at the lower end of the recommended dose range were superior to placebo” and “inferior to higher doses”, suggesting a linearly increasing dose-efficacy relationship for SSRIs. Within their highdose categories corresponding with citalopram 40–60 mg, paroxetine 20–40 mg, or sertraline 100–200 mg, they found no indication of dose dependency. However, their category of low dose included subtherapeutic doses (citalopram 10 mg or paroxetine 10 mg) as well as therapeutic doses (citalopram 20 mg and sertraline 50 mg), which might explain why the low-dose category did less well than the high-dose category in their analysis.
In 2016, Jakubovski and colleagues (
7) found a statistically significantly greater response for high doses in a meta-regression analysis of 40 studies comparing SSRIs with placebo (n=10 039); an accompanying editorial (
27) concluded, “there is a modest but clear dose-response effect for SSRIs.” However, when the findings were categorised into less than 20 mg, 20–39 mg, 40–50 mg, and more than 50 mg fluoxetine equivalents, the authors found the 40–50 mg dose category offered the greatest efficacy (ie, there was no general linear relationship between dose and efficacy over the whole dose range). Moreover, a majority of their included studies were flexible-dose studies and they took the maximum of the flexible range as the dose representing the treatment groups.
Our dose-dependency curves were based on models using splines, which treated dose as a continuous variable, and shed new light on the existing evidence. Our results for dose-efficacy relationships for SSRIs are largely in line with Bollini and colleagues (
5). Hieronymus and colleagues’ (
6) observation that there was a stepwise increase in efficacy from placebo, to low dose, to higher dose of SSRIs might be considered compatible with our finding of dose response up to 20–40 mg of fluoxetine equivalents; however, their categorisation of doses did not capture the change points identified in our analyses. Jakubovski and colleagues’ (
7) conclusion that 40–50 mg of fluoxetine equivalents offered the greatest efficacy might be overstated if we take into account that most of their included studies used flexible-dose regimens and the prescribed doses could be significantly lower than 40–50 mg.
Two studies examined the relationships between doses and side-effects. Bollini and colleagues (
5) found a monotonic increase in adverse event rates, from 20% to 50%, as the dose increased, from zero to more than 50 mg fluoxetine equivalents. Jakubovski and colleagues (
7) found a monotonic dose relationship for dropouts due to side-effects, but an almost flat relationship for all-cause dropouts. These findings might be confounded by the inclusion of both fixed-dose and flexible-dose studies. By contrast, by focusing on fixed-dose studies and using flexible spline curves, we found a linear to exponential relationship between dose and dropouts due to side-effects, and a curvilinear relationship between dose and all-cause dropouts for SSRIs.
Our study is not without limitations. First, the findings mainly pertain to patients with major depression who were judged eligible for placebo-controlled trials. For patients who suffer from physical comorbidities or for older (ie, age ≥65 years) patients, the optimal dose might be lower than 20–40 mg fluoxetine equivalents. In this context, the positive dose-efficacy association through zero to 30 mg of fluoxetine equivalents is clinically relevant. Second, the fixed-dose regimen might be regarded as not reflecting clinical practice, especially when no or rapid titration scheme is used, and might overestimate withdrawal due to adverse effects. Tolerability might then confound efficacy because interventions with high dropouts are likely to show lower endpoint efficacy because the majority of patients leave the study early and therefore have less time to improve; however, strict examination of dose dependency is possible only with fixed-dose studies. Third, there is some uncertainty about how best to calculate dose equivalency among antidepressants. We used the most comprehensive empirically derived conversion algorithms (
16), which were similar to the ones used in previous systematic reviews on this topic (
5,
7). We tested our results via sensitivity analyses that used alternative conversion algorithms and confirmed the primary results. Fourth, spline curves for individual drugs were based on a few studies, resulting in wide CIs. We therefore meta-analysed all SSRIs because they share a key therapeutic mechanism. The curves for individual SSRIs provided little evidence of heterogeneity. For venlafaxine and mirtazapine, the numbers of available trials were few and the resultant wide CIs, particularly regarding tolerability and acceptability, warn against overinterpreting the results. Fifth, we used dropouts for side-effects from the acute-phase treatment as an index of tolerability: these numbers do not necessarily reflect rare but severe adverse events, nor do they include long-term side-effects, including withdrawal symptoms. Lastly, the search date for the relevant studies could be considered old; however, an update search for eligible trials in PubMed on March 21, 2019 revealed only one additional eligible study with a small sample size (55 patients randomly assigned to paroxetine 10 mg and 49 to placebo) (
28), which would not change the results of our analysis with 19 364 patients.
Our study has a number of strengths. First, we used state-of-the art dose-response meta-analysis, and treated dose as a continuous variable, allowing greater resolution of change points and avoiding misleading categorisation of doses. Second, we examined dose dependency not only for efficacy but also for tolerability and acceptability. Third, our study is based on the largest and most comprehensive dataset of fixed-dose, double-blind, randomised controlled trials, published or unpublished, of second-generation antidepressants in the acute-phase treatment of major depression. The included numbers of studies and participants were two to eight times larger than the previous analyses. We analysed not only SSRIs, but also two widely prescribed non-SSRI antidepressants, venlafaxine and mirtazapine. Our findings will have clinical implications, especially for practitioners or countries where fluoxetine is routinely prescribed at doses of 40 mg or more.
In conclusion, our analyses showed dose dependency in efficacy up to around 20–40 mg fluoxetine equivalents, beyond which there is no further increase in efficacy for SSRIs or mirtazapine, but a possibly slight increase for venlafaxine; a clear dose dependency in dropouts due to adverse effects for all drugs through the examined dose range; and the overall acceptability of treatments appears to be optimal towards the lower end of the licensed range. We therefore conclude that for the majority of patients receiving an SSRI, venlafaxine, or mirtazapine for the acute-phase treatment of their major depressive episode, the lower range of the licensed dose will probably achieve the optimal balance between efficacy, tolerability, and acceptability. Clinical guidelines need to incorporate these findings.