Schizophrenia is a debilitating psychiatric illness that is among the world's top 10 causes of long-term disability. The symptoms of schizophrenia include psychosis, apathy and withdrawal, and cognitive impairment, which lead to problems in social and occupational functioning and self-care. Approximately 0.7% of the population is affected by schizophrenia, with similar rates across different countries and cultural groups (
1,
2). Mental health care in indigenous populations in Latin America is markedly constrained by the scarcity of mental health resources, as well as by the gap in knowledge about their cultural health practices and the forms of expression of mental symptoms in their cultural context (
3–
5). Schizophrenia affects people who are just entering the peak of social, economic, and intellectual productivity. People affected by schizophrenia withdraw; fail to establish meaningful relationships with peers, relatives, or life partners; and fail to reach the expected level of social function. Such functional loss is particularly relevant in indigenous communities, which rely on change in functional status (rather than on the presence of symptoms) to identify mental illness (
6). Particularly among the indigenous communities of Latin America, the gap between mental health need and availability of resources to reduce the burden has been judged “a case of outrageous exclusion” (
3). The World Health Organization (WHO) proposed an action plan to scale up services giving priority to schizophrenia along with other burdensome disorders (
7); the plan included a set of interventions including “task-shifting” strategies as a rational redistribution of tasks among health workforce teams (
7).
For more than a decade, as part of the Investigation of Movement Abnormalities and Genetic of Schizophrenia (IMAGES) study, our group has been studying vulnerability markers (genetic, motor, imaging, and neuropsychological differences) for schizophrenia in a remote, indigenous population in rural northern Argentina (
8–
10). At the center of the IMAGES strategy was the implementation of a task-shifting paradigm in which recognition and engagement of “index” cases were accomplished by specifically providing training for primary care health workers, leading to effective interventions coordinated at local primary care delivery systems (
9). The most striking success of this strategy was that because ongoing training of health agents resulted in more proficient identification and referral of individuals with untreated psychosis, a severalfold reduction in the duration of untreated psychosis was observed across the entire province (
9). A longer duration of untreated psychosis is a well-established predictor of poorer outcomes (
11,
12) and may result in reduced cortical thickness (
13) and cognitive impairment (
14), although the latter was disputed recently (
15,
16). A recent meta-analysis confirmed that a longer duration of untreated psychosis predicts poor general symptomatic outcomes, more severe positive and negative symptoms, lesser likelihood of remission, and poor social functioning and global outcomes (
17). Thus, reducing the duration of untreated psychosis severalfold, as our study shows is possible through a task-shifting strategy, has the potential to greatly affect the burden of disease in lower- to middle-income communities and improve the trajectory of psychosis.
Identification of Proxy Markers of Risk for Schizophrenia in a Primary Care Environment
Because of geographical isolation and a lack of distributed mental health services, people in the Andean region are unlikely to come into contact with mental health specialists during much of the course of their illness. Therefore, we reasoned that in addition to posing a public health deficit, their situation offered a unique scientific opportunity to study disease processes without interference from the effects of medications at the time of detection. One of the main findings from this investigational effort is that neuroleptic-naive patients with schizophrenia have increased hyperechogenicity of the substantia nigra (a marker for parkinsonism) and an associated increase in motor symptoms of parkinsonism (
10). Furthermore, the same abnormalities, albeit to a less severe degree, were found in their unaffected first-degree relatives (
10). Although it has been well established that antipsychotic drugs can lead to parkinsonism (
18,
19), it is worth pointing out that the presence of parkinsonism in untreated schizophrenia is not a novel finding. Kraepelin (
20) described identical movement abnormalities in patients with schizophrenia more than a century before the introduction of neuroleptics, and parkinsonism has been found repeatedly in diverse populations of neuroleptic-naive patients with schizophrenia, including first-episode (
21–
25) and chronically ill patients (
10,
26). In high-risk populations, delayed motor development and impaired motor skills have been reported in individuals who went on to have schizophrenia (
27–
29), and an increased rate of parkinsonism in first-degree relatives of patients with schizophrenia has also been reported (
10). These studies suggest that motor abnormalities may be an integral part of the pathogenic process of schizophrenia and may serve as indicators of vulnerability to the disorder.
However, neuromotor precursors are subtle and may lack sufficient specificity to be considered (at least by themselves) as proxy markers for primary prevention (
30), which is unquestionably the goal to be strived for in resource-poor environments where treatment options may be severely limited and where the burden of disease is greatest. Neuroimaging techniques offer great promise to identify proxy markers of risk (
31), but their cost and complexity generally rule out their use as screening instruments at the population level, particularly in rural environments such as the Andes. On the other hand, transcranial ultrasound screening is performed with a noninvasive, affordable, portable device with minimal support requirements and broad availability, which can be used in primary care rural environments. Given the well-established findings using this technique in the midbrain parenchyma of patients with idiopathic Parkinson’s disease, in which increased echogenicity of the substantia nigra is a marker of disease (
32) and of disease risk (
33), we reasoned that its use could lend itself as a biomarker of parkinsonian motor impairment in patients with schizophrenia and as a risk marker in their unaffected relatives. Indeed, hyperechogenicity of the substantia nigra correlates directly with the severity of antipsychotic-induced extrapyramidal side effects in patients with chronic schizophrenia (
34,
35).The cause of hyperechogenicity of the substantia nigra has not been established, but it is associated with iron accumulation related to neuronal damage (
36) and with reduced [18F]-dopa uptake in the striatum in patients with premotor Parkinson’s disease (
37). Thus, increased echogenicity of the substantia nigra may be associated with an impairment of the dopaminergic nigrostriatal system. Notably, we recently reported that in our isolated, indigenous population of Argentina, hyperechogenicity was present and predicted parkinsonian motor impairment in patients with schizophrenia who were never exposed to neuroleptics and in their unaffected first-degree relatives (
10), indicating that midbrain abnormalities along with movement abnormalities are inherent parts of the underlying pathophysiology and may serve as markers of risk.
Methods
Study Population and Design
As part of the IMAGES study, health care agents in the province were instructed to notify the provincial epidemiology system upon detection of cases of untreated psychosis. From 2004 to 2015, a total of 62 patients with chronic untreated schizophrenia were evaluated and engaged in care by the public health system. Assessment consisted of semistructured interviews with the WHO Schedules of Clinical Assessment in Neuropsychiatry (SCAN); if criteria for a diagnosis of schizophrenia were met, motor assessment was obtained with the Unified Parkinson’s Disease Rating Scale Part III (UPDRS-3) and a transcranial ultrasound. We only included participants who were aged 18–65 years, who had no comorbid neurological or psychiatric disorders or substance abuse disorders, and who were able to provide informed consent. All other participants who came into contact with the mental health specialists through health agent referrals were also engaged in treatment free of charge regardless of diagnosis or age, but they were not followed-up prospectively. Once engaged in care, participants were treated by nonstudy community providers using standard-of-care criteria. After varying lengths of treatment ranging from 3 to 11 years, all participants were invited to participate in the current follow-up study. We re-evaluated 43 participants (26 men and 17 women) using the Positive and Negative Syndrome Scale (PANSS), the Clinical Global Impressions Scale (CGI), and the UDPRS-3. In addition, we carried out chart reviews to evaluate medication history and to calculate dose-years (a measure of cumulative neuroleptic exposure). Three patients refused to participate, three patients were deceased, and the rest were unreachable. Thus, 70% of original patients were re-evaluated in the follow-up portion of the study. All protocols used in this study were approved by the Ethics Committee of the Province of Jujuy and by the Morsani College of Medicine Institutional Review Board at the University of South Florida.
Transcranial Ultrasound Screening
Protocols for a transcranial ultrasound examination were published previously (
10). Briefly, we utilized a color-coded, phased-array ultrasound system, equipped with a 2.5-MHz transducer (Micromaxx; Sonosite Inc., Bothell, WA). Examinations were performed through a preauricular acoustic bone window (penetration depth=16 cm, dynamic range=45 dB) by an expert sonographer with more than 20 years of experience with the technique who was blinded to the patient’s condition. Less than 3% of cases were not suitable for transcranial ultrasound screening because of skull thickness (a much lower percentage of that reported in the Parkinson’s disease population, most likely because our patients are much younger). The substantia nigra was identified within the butterfly-shaped structure of the brainstem, scanning from each temporal bone window. Because the signal brightness (echogenicity) is not quantifiable by an ultrasound, the area of hyperechogenic signals in the substantia nigra region was measured (in centimeters squared) from each ipsilateral side separately, and unbiased quantification of the echogenic area was carried out post hoc on saved images by two different evaluators blinded to the patient’s condition.
Clinical Assessments
Diagnostic ascertainment was carried out with the SCAN 2.1 (WHO, Geneva) scored blindly by two independent raters at the initiation of the study. The SCAN is a set of instruments and manuals aimed at assessing, measuring, and classifying psychopathology and behavior associated with major psychiatric disorders in adult life. It can be used for clinical, research, and training purposes and was developed within the framework of WHO. The SCAN has a bottom-up approach in which no diagnosis-driven frames are applied in grouping the symptoms. Each symptom is assessed in its own right. It has a proven stability and robustness to differentially assess psychotic states. Interview data are entered into the program and are fed into algorithms for ICD-10 and DSM-IV diagnoses. These algorithms produce a diagnostic classification for both systems. To meet selection criteria, all participants met the criteria for DSM-IV schizophrenia when scored by two independent raters.
Motor assessment of parkinsonism in the initial phase of the study was scored blindly using the UPDRS-3 (
38,
39) on videotaped examinations by two independent raters certified in its use. Thus, the diagnosis of parkinsonism was not based on the UK Brain Bank criteria but on an arbitrary cutoff point of the UPDRS-3. Parkinsonism at the time of the follow-up phase of the study was also scored blindly using the UPDRS-3 by raters certified in its use.
Clinical assessments used to measure psychopathology were the PANSS for schizophrenia (
40) and the CGI severity scale (
41). The PANSS assesses 30 different symptoms on a scale from 1 to 7 based on a clinical interview. For this study, total scores as well as positive and negative subscores were examined. The CGI severity scale rates the severity of the patient’s mental illness from 1 (normal, not at all ill) to 7 (extremely ill).
To measure cumulative neuroleptic exposure, we computed the dose-years (
42), after conversion of all antipsychotic agents to chlorpromazine equivalents using published tables for conventional and atypical antipsychotic agents (
43,
44). A dose-year is defined as the product of the chlorpromazine equivalents and the time on each dose expressed in years (
42).
Statistical Analyses
Independent-measures t tests were used to measure the effect of gender on baseline characteristics (age, length of treatment, percentage treated with clozapine) as well as experimental measures (UPDRS-3, CGI, PANSS, right and left substantia nigra ultrasound, and dose-years). The independent-measures t test was also used to measure differences in clinical and motor outcomes in patients treated with clozapine versus those treated with other antipsychotic agents. To determine predictors of outcome (baseline transcranial ultrasound measurements, baseline parkinsonism, and age), multiple backward correlation analyses were calculated for follow-up measurements (PANSS, CGI, dose-years, and postmedication UPDRS-3 score), each as dependent variables. All analyses were performed with the IBM SPSS statistical package, version 23.
Discussion
This study is part of a larger multidisciplinary collaborative effort designed to tackle the burden of severe mental illness in Latin America. Participants were recruited, monitored, and followed-up through a task-shifting paradigm that relies on community health agents as screeners and ongoing evaluators, with psychiatrists and mental health specialists functioning only as supervisors and consultants in designing and providing training for the interventions. This approach resulted in a severalfold decrease in the duration of untreated psychosis (
9) and in very high retention rates (70%) and treatment adherence over a decade. Indeed, although formalized outcome measurements were not carried out until now, the majority of patients have had regular home visits by health agents for medication management and periodic follow-up visits at the regional hospital for medication adjustments. Blood draws for safety monitoring of clozapine are also carried out at the local health posts by the health agents, and these tests are submitted to the regional hospital for analysis. Our retention rate of 70% through a decade (43 of 62 patients) is acceptable and consistent with published data in population-based studies with much shorter follow-up times in developed countries (
45). With this collective effort involving health care workers and a primary psychiatrist, patients were successfully followed in the clinical setting, and treatment adherence, treatment response, predictors of therapeutic response, and levels of functioning were all obtained many years after initial recruitment into the study.
Several limitations must be pointed out, including the skewed gender distribution (i.e., twice as many male than female index cases), overall small sample size (N=43), as well as a varying amount of years in study participation (from 3 to 11 years). Another topic to consider is the possible influence of culture on symptom profiles, course, and outcome of schizophrenic disorders. Two international collaborative research projects undertaken by the Mental Health Division of WHO, namely the International Pilot Study of Schizophrenia and the study of the Determinants of Outcome of Severe Mental Disorders, surveyed patients and outcomes at 12 research sites in diverse sociocultural settings (Colombia, Czechoslovakia, Denmark, India, Ireland, Japan, Nigeria, Russia, the United Kingdom, and the United States) and revealed that syndromes of schizophrenia occur in all cultures and geographical areas investigated and that their rate of incidence is very similar in the different populations (quite a narrow range between 0.1 and 0.4 per 1,000 population) (
46,
47). In WHO international studies of schizophrenia, patients in Western developed countries showed a higher frequency of depressive symptoms, primary delusions, thought insertion, and thought broadcasting than those in non-Western developing countries, who instead endorsed visual and directed auditory hallucinations more frequently (
46,
47). Remarkably, however, although the content of delusions and hallucinations varies from culture to culture, the form of the illness remained very similar around the globe (
47). We used the same standardized instrument as the two large-scale WHO studies: the present state examination (SCAN), which has shown strong reliability in this Andean population (
9). The reliability of the SCAN is well established, and the instrument has been used extensively in cross-cultural studies. It has been translated into approximately 20 languages and field tested in a dozen countries (whoscan.org). Thus, although subtle differences were indeed noticeable regarding the specific content of delusions, these are unlikely to affect the results of diagnoses based on the Present State Examination–SCAN, because the overall symptom profile of the disease was indistinguishable from descriptions of chronic schizophrenia throughout the world.
The prospective analysis of outcome predictors highlights two main findings: namely, that left substantia nigra hyperechogenicity at the time of diagnosis predicts negative symptoms and parkinsonism at follow-up, and that right-sided parkinsonism at diagnosis significantly predicts a more severe course of illness (
Table 2). With respect to the predictive value of ultrasound findings for motor deficits, this is in line with several correlational studies reporting an association with hyperechogenicity of the substantia nigra and parkinsonism in nonmedicated patients with schizophrenia (
10) and in acutely and chronically treated patients with schizophrenia (
34,
35). Of note, the transcranial ultrasound findings and motor deficits in treatment-naive patients with schizophrenia were not identical to ours, but the overall implications were very similar. Specifically, participants with untreated schizophrenia have more left-sided motor deficits and simultaneous contralateral right substantia nigra abnormalities on an ultrasound at the initial diagnosis. After treatment, these asymmetries become less apparent. Nonetheless, patients with schizophrenia before and after treatment have significant parkinsonism and more substantia nigra hyperechogenicity, which correlate with course severity. Indeed, to our knowledge, this study is the first to show that substantia nigra hyperechogenicity can serve as a marker for adverse motor and clinical outcomes many years later.
With respect to the predictive value of hyperechogenicity for ongoing negative symptoms and parkinsonism in our sample of patients, our findings are again consistent with previously published reports of parkinsonism in treated patients and are associated with negative symptoms (
4), a combination described as a ‘‘negative movement disorder’’ (
48). The presence of extrapyramidal side effects correlated with more negative symptoms and poorer treatment outcomes that were reflected in a longer time to, and lower level of, remission (
48). On the other hand, in one study of patients who were not medicated, the relationship between psychopathology and extrapyramidal side effects could not be established (
26).
Neither age of illness onset nor premedication parkinsonism in our sample was predictive of postmedication parkinsonism, psychopathology, or cumulative drug exposure. Indeed, the relationship between age and extrapyramidal side effects is controversial in the literature. In nonmedicated patients, no correlation between extrapyramidal symptoms and age at assessment or at onset of psychotic symptoms was found (
23,
26). Conversely, in medicated patients, the risk for dyskinesia increased with age (
49) and duration of illness (
50). In chronically treated patients with schizophrenia, hyperechogenicity correlates with age and severity of parkinsonian symptoms but not with dose or type of antipsychotic agent (
35). In our study, the majority of patients were younger than 25 years at illness onset, and the narrow distribution of ages would make it difficult to detect an effect of age on multiple outcome variables, given our relatively young population.
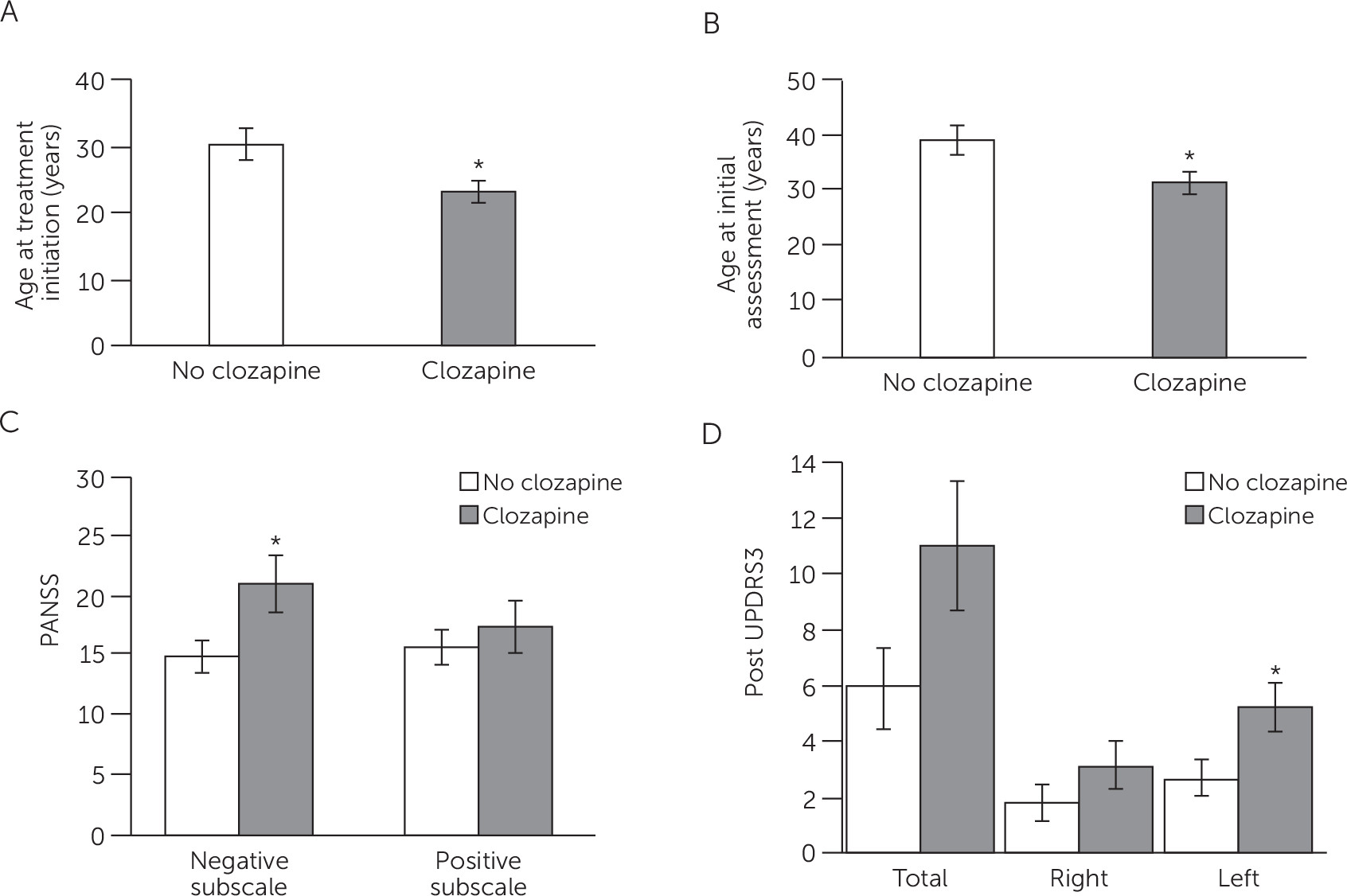
Clozapine improves outcome in patients with schizophrenia in whom typical antipsychotic medications are ineffective or are not tolerable (
51) and results in lower incidence of extrapyramidal symptoms, which are the best predictor of quality of life (
52). Roughly one-half of our patients were treated with risperidone, aripiprazole, chlorpromazine, levomepromazine, or haloperidol, whereas the rest were switched to clozapine after two failed medication trials with other antipsychotic agents. We examined differences in age of illness onset, treatment duration, substantia nigra hyperechogenicity, and parkinsonism in clozapine-treated patients versus those treated with other antipsychotic drugs. Patients treated with clozapine were younger at onset, had more negative symptoms, and had more left parkinsonian motor impairment at follow-up. There are several explanations for increased parkinsonism in those treated with clozapine, including clozapine side effects, fluctuations of extrapyramidal symptoms with time (
53), or more parkinsonism overall in patients refractory to treatment, independent of medication side effects. These findings support the regression analysis data showing that worse parkinsonism and negative symptomatology are predicted by left substantia nigra abnormalities, which may be an indicator of overall severity of illness and eventual treatment with clozapine.
In summary, parkinsonism is common in neuroleptic-naive patients with schizophrenia, and patients with more severe motor impairment are likely to be more susceptible to extrapyramidal side effects from antipsychotics, leading to treatment refractoriness (
54) and to a higher likelihood of clozapine use (
52,
55). Thus, we propose transcranial ultrasound screening and testing for parkinsonism at illness onset before the introduction of neuroleptics as potentially useful markers in determining illness severity, negative symptomatology, and tolerance to antipsychotic treatment/refractoriness. Transcranial ultrasound screening may help to identify a subset of patients with worse clinical course and outcome. This subset could be considered for clozapine earlier in the course of illness, eliminating the costly process of determining resistance, treatment withdrawals, hospital readmissions, and severe side effects.
Our experience in a rural indigenous population of the Andean region has shown the feasibility of building teams of local researchers. These teams, by working on a rigorous state-of-the-science research program, have also had an immediate, lasting, and measurable impact on decreasing barriers of the most vulnerable and underserved populations to mental health care, and they have modified local practices by incorporating mental health surveillance practices into primary health strategies as well as by fostering early detection of severe mental health disorders. The regional teams for multidisciplinary collaborative research reflect the necessary range of expertise required for tackling research and treatment of the complex etiology of psychiatric and behavioral disorders.