Overview
Cognitive deficits are a core feature of depression among both adults and elders, which makes depression a “cognitive disorder” for many people (
1). Cognitive deficits are consistently found in the domains of memory, verbal learning, motor speed, executive functioning, and processing speed (
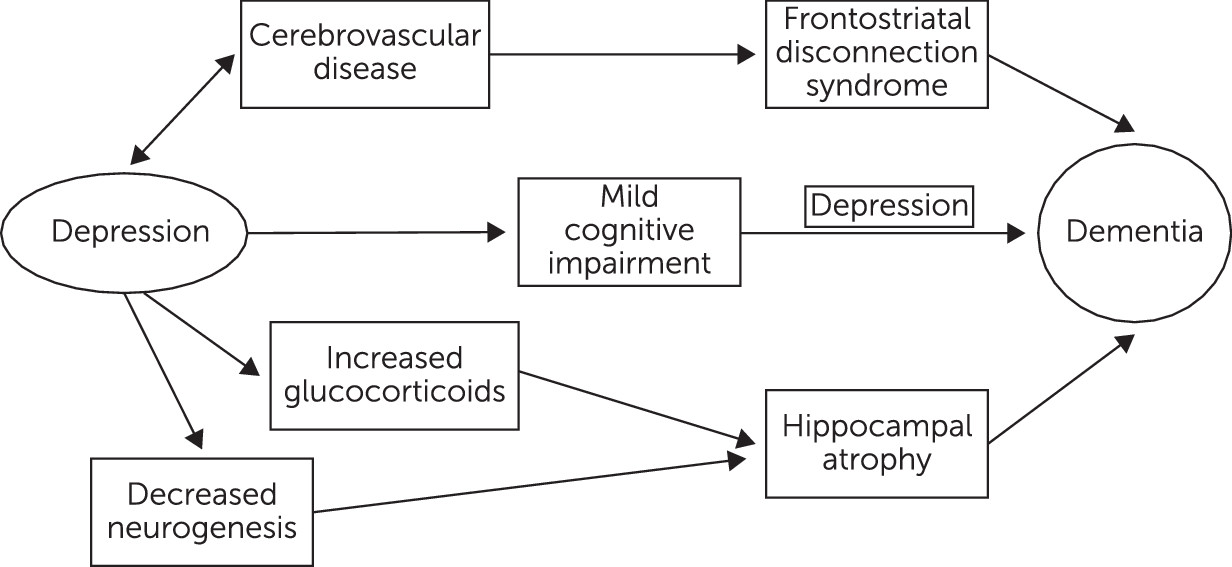
2). In the past, such deficits were thought to be temporary and isolated to periods of dysphoria. They were labeled “pseudodementia” or “reversible dementia.” Increasingly, there is evidence to suggest that cognitive deficits persist despite remission of depression. Continuing cognitive deficits may be related to underlying brain changes, including hippocampal atrophy, alterations in glucocorticoid secretion, and cerebrovascular compromise (
3–
5). Other biological mechanisms linking depression to cognitive impairment include increased deposition of β-amyloid plaques, chronic inflammation, and deficits of nerve growth factors, such as brain-derived neurotrophic factor (
6).
Fifteen years ago, Jorm (
7) proposed three hypotheses to explain the relationship between dementia and depression. Depression can, first, be an early prodromal sign of dementia; second, bring forward the clinical manifestations of dementing disease; and third, lead to hippocampal damage through a glucocorticoid cascade. He concluded that depression is likely a risk factor for dementia in general and Alzheimer’s disease specifically (
7).
This article appraises several facets of the linkage between depression and cognitive impairment, including whether depression is a risk factor or a prodromal feature of dementia. Other areas of exploration include the impact of midlife, late-life, and vascular depression on cognition and possible mechanisms whereby depression may result in lasting cognitive impairment. This review was crafted to be extensive, although not exhaustive, because this is a rapidly expanding area of inquiry.
Description
Depression among older adults can have a variety of presentations. It may arise as recurrent disease stemming from earlier life. This has been termed early-onset or midlife depression. Late-onset or late-life depression is described as depression that initially occurs past the age of 60 to 65 years. Depression can also occur as a mood disorder secondary to another medical condition or as mood symptoms secondary to substance use or medication.
Symptomatic differences have been noted in early-onset depression and late-life depression. Early-onset depression has been characterized by greater personality abnormalities, a family history of psychiatric illness, and dysfunctional past maternal relationships. The mood is often depressed, hopeless, and accompanied by suicidal thoughts (
8). Late-life depression has been described by fearfulness, poor appetite, weariness, hopelessness, worthlessness, guilt, and anger. People with late-life depression have fewer crying spells and less sadness (
8,
9). Anxiety, somatic complaints, and thoughts of death, rather than outright suicide ideation, may occur more frequently (
9).
As noted, cognitive deficits are a core feature of depression for both adults and elders. Among younger adults, decreased concentration and indecisiveness are seen, whereas cognitive complaints of older adults may include selective attention, problems in working memory, impaired memory retrieval, difficulties in learning, slowed processing speed, and executive dysfunction (
10–
12).
Table 1 summarizes these presentations. Cognitive deficits persist even during euthymic periods, and, compared with healthy others, euthymic patients postdepression have had significantly poorer cognitive function (
11). Cognitive deficits seem to be more common among patients with late-onset depression, compared with those with early-onset disease. This supports theories suggesting that vascular and neurodegenerative factors, which are more common in late life, affect a substantial number of these patients (
11) and that etiologically, late-life depression may be different from early-onset depression.
Depression and Dementia
The crux of the association between depression and dementia has focused on whether depression is a risk factor for dementia, a prodrome of dementia, or some combination of both. The majority of published studies have highlighted an increased risk of dementia among people with previous diagnoses of depression. However, approximately one quarter of studies have not shown any statistically significant increase in this risk (
15), which has led to continued uncertainty regarding this association. Other areas of inquiry have been whether there is differential risk for dementia among patients with early-onset, late-life, vascular, or recurrent depression.
Cognitive deficits can persist despite remission of depressive symptoms, which indicates that depression may have a longer term pernicious effect on cognition. In one study, conducted over three years, irreversible dementia developed significantly more frequently among depressed elders with reversible dementia or pseudodementia (43%) than among the group with depression alone (12%). The group with reversible dementia had a 4.69 times higher chance of developing dementia than patients with depression alone (
16). Cognitive areas affected included visuoperception, processing speed, and memory function (
13). Researchers have continued to find deficits up to 18 months and four years later postdepression, so that cognitive impairment among elderly patients with depression may be a trait characteristic, such that even when depression has remitted, cognitive impairment persists (
3).
With respect to depression as a risk factor for dementia, data have been mixed (
7,
17). A large systematic review and meta-analysis concluded that depression was a risk factor for dementia. The researchers appraised 20 studies involving 102,172 patients. They separated case-control studies, which are retrospective and subject to recall bias, from cohort studies, which are prospective. The authors found calculated pooled odds ratios (ORs) of 2.03 for case-control studies and 1.90 for cohort studies. The interval between diagnoses of depression and Alzheimer’s dementia (AD) was positively related to increased risk of developing AD, which suggests that rather than a prodrome, depression may be a risk factor for AD (
18). Another meta-analysis involving older adults and elders over age 50 found that depression was also associated with a significant risk of all-cause dementia (OR=1.85), AD (OR=1.65), and vascular dementia (VaD; OR=2.52) (
14).
The hypothesis that depression is a prodrome to dementia is most compatible with studies in which depression occurs near the onset of dementia. The MIRAGE study was a case-control study involving more than 4,000 individuals. A significant association was found between depression and AD, with an OR of 2.13. Where depression first occurred within one year before onset of AD, the OR was 4.47. Where depression first occurred more than one year before onset of AD, the OR was 1.38. Where depression first occurred more than 25 years before onset of AD, the OR was 1.71 (
19). The data suggested that depression is a significant prognostic indicator for dementia, as well as a risk factor. Barnes et al. (
20) conducted a retrospective cohort review of 13,535 Kaiser Permanente patients with an average age of 81.1 years. Depressive symptoms were present among 14.1% of patients in midlife only, 9.2% in late-life only, and 4.2% in both. During six years of follow-up, 22.5% of the patients received a diagnosis of dementia. The adjusted hazard ratio of dementia was increased by approximately 20% for midlife depressive symptoms only (OR=1.19), 70% for late-life symptoms only (OR=1.72), and 80% for both (OR=1.77). When AD and VaD were examined separately, patients with late-life depressive symptoms had a twofold increase in AD risk (OR=2.06), whereas patients with midlife and late-life symptoms had more than a threefold increase in VaD risk (OR=3.51). In this analysis, late-life depressive symptoms were more predictive of dementia than early-life depressive symptoms (
20). Other studies have also found that a history of current depression and increasing depressive symptoms in late life increase the risk of cognitive impairment, whereas a history of past depressive symptoms or low depression is not associated with cognitive impairment. These data suggest that depression in later life may also represent a prodromal phase of cognitive impairment (
21,
22).
Recurrent depression seems to present a greater risk for dementia than single-episode depression, such that patients with many prior hospitalizations for depression have an increased risk of developing subsequent dementia. On average, each depressive episode has been associated with a 13% increased rate of dementia (
23). This result has been confirmed in a prospective study of 1,239 older adults from the Baltimore Longitudinal Study of Aging. In that study, each depressive episode was associated with a 14% increase in risk for all-cause dementia (
24).
In sum, the proposals of depression as either a risk factor or a prodrome for dementia are not mutually exclusive. Data currently seem to reflect that depression is both a risk factor and a prodrome for dementia (
15,
18–
20,
25,
26), although there is evidence to the contrary (
21,
27–
29), likely because of heterogeneity in methodology, populations, and other factors. More data support early-onset depression’s serving as a risk factor for dementia (
17,
30) than support late-life depression’s acting as a prodromal symptom of dementia (
20,
21). However, in studies looking across age ranges, the magnitude of risk ratios decreases with the time between depression onset and dementia diagnosis (
1,
19,
20), which makes it more likely that later onset of depression may be a more significant prodromal symptom or risk factor for later dementia (
1). There also seems to be a dose-response relationship between depression and dementia, such that each depressive episode is associated with an increased rate of dementia (
23,
24).
Depression and Mild Cognitive Impairment
The term
mild cognitive impairment (MCI) is used to characterize nonnormal cognitive function that is not severe enough to meet diagnostic criteria for dementia (
1). It has been conceptualized as a predementia state or a warning sign that the risk of dementia is high. Depression occurs commonly among people with MCI and has been found in 30%−45% of patients with MCI (
31,
32). More than 20% of patients with MCI convert to dementia within a three-year period (
33). However, the majority of MCI patients do not convert. Researchers have attempted to determine which factors lead to dementia conversion and have looked at depression, apolipoprotein E (APOE) genotypes, and amnestic MCI (aMCI).
Studies have shown that the presence of depressive symptoms increases the rate of conversion of MCI to dementia (
34). In one study of MCI patients, after a mean period of three years, 85% of the patients with depression developed dementia, in comparison with 32% of the patients without depression. The relative risk (RR) was 2.6. Patients with MCI and depression had more than twice the risk of developing AD as those without depression (
32). This finding was confirmed in a large meta-analysis. Eighteen studies were included, with a sample size of 10,861 patients with MCI. The pooled RR of progressing to dementia was 1.28 for the group of MCI patients with depressive symptoms, compared with nondepressed patients with MCI (
35).
Other factors that might affect the role of depression in conversion of MCI to dementia include APOE status and the presence of aMCI. The risk of developing AD has been significantly higher among depressed aMCI groups than among depressed nonamnestic groups (
36). The role of APOE status in conversion to dementia and the development of MCI has been more mixed. In one study, 21% of depressed elders met criteria for aMCI. Among depressed elders, aMCI and age were most associated with incident dementia and AD. Any APOE ε4 allele was not significant (
37). Another study found significance only for depression and ε3/ε4 or ε4/ε4 genotypes in the development of MCI (hazard ratio=5.1) (
38).
Researchers have used imaging studies to further explore the relationship between depression and MCI. A synergistic effect of depressive symptoms and smaller left hippocampal volume among MCI patients has been found to accelerate conversion to dementia (
39). In the Alzheimer’s Disease Neuroimaging Initiative study, those with persistent depressive symptoms had higher conversion to AD, compared with those who remained without neuropsychiatric symptoms. Patients with MCI who had comorbid depressive symptoms showed more frontal, parietal, and temporal white matter atrophy than asymptomatic patients (
40).
Vascular Depression and Dementia
The vascular depression hypothesis postulates that cerebrovascular disease predisposes, precipitates, or perpetuates a depressive syndrome among some older adult patients (
4). The hypothesis is supported by the known comorbidity of depression, vascular disease, vascular risk factors, and ischemic lesions with distinctive behavioral symptoms. Researchers have postulated that the central mechanisms are disruption of prefrontal systems and their limbic and hippocampal connections or their modulating pathways by single lesions or by an accumulation of lesions exceeding a threshold (
39).
Patients with vascular depression have characteristic features. With respect to clinical symptoms, patients have significant psychomotor retardation, little psychomotor agitation, less guilt, poorer insight, and limited depressive symptoms, compared with healthy others (
41). Individuals have tended to be older, have higher levels of hypertension and other cerebrovascular risk factors, and have less family history of depression (
42,
43). They also have greater overall cognitive impairment and disability than those with nonvascular depression. Cognitively, fluency and naming are impaired (
4,
41). However, the core cognitive deficits in late-life depression seem to be slowed processing speed, followed by executive dysfunction. Changes in processing speed most fully mediate the effect of age, depression severity, education, race, and vascular risk factors on other cognitive domains, including episodic memory and visuospatial performance. These declines persist even after remission of depression (
44,
45).
Supporting the vascular depression hypothesis, a large proportion of elders with depression have had either a stroke or other evidence of cerebrovascular compromise (
41,
46,
47). Pooled data from studies have found poststroke prevalence rates for major depression of 19.3% among hospitalized patients and 23.3% among outpatients (
47). These numbers are higher than the prevalence of major depressive disorder in the general population (8.3%) (
48).
High rates of cerebrovascular disease, along with white matter hyperintensities (WMHs) on MRIs, have also been found among depressed elders (
49). WMHs are thought to be caused by small, silent cerebral infarctions. They are characterized by arteriosclerosis, perivascular demyelination, dilated periventricular spaces, and ischemia (
50,
51). They can predispose individuals to depression (
49,
52) by disrupting the fiber tracts connecting cortical and subcortical structures (
52) in areas such as the dorsolateral prefrontal cortex and the anterior cingulate cortex (
50). In one study involving patients with late-life depression, silent cerebral infarctions were observed among 65.9% of those with early-onset depression and among 93.7% of those with late-life depression (
53). A systematic review (
54) confirmed this finding. The authors found that late-life depression was characterized by more frequent and intense white matter abnormalities than early-onset depression. The odds of having white matter changes were over 4 for late-onset illness, compared with early-onset illness. These differences suggest different etiological mechanisms for late-life depression, in keeping with the vascular depression hypothesis (
54). Last, considering structural brain changes, Kramer-Ginsberg et al. (
55) found that among elders with depression, hyperintensity location/severity and the presence or absence of depression had a significant interaction with cognitive performance. Depressed patients with moderate-to-severe deep WMH had worse performance on general and delayed recall memory, executive function, and language testing than depressed patients without WMH and normal elders with or without deep white matter changes. (
54).
Regarding vascular depression and vascular dementia, the similarity of risk factors and findings for the two conditions implies a connection. Both conditions encompass cerebrovascular compromise through vascular risk factors, ischemic lesions, and the detection of WMHs, among other potential findings. However, the path from vascular disease to vascular depression to vascular dementia has not been elucidated. Furthermore, the road may be reciprocal and not direct or sequential, given that depression can also increase the risk of vascular disease and vice versa (
4). Unfortunately, research linking vascular depression to dementia, including vascular dementia, is limited (
27,
56). However, it is likely that a connection exists between these conditions and that structural brain changes may be a distinctive feature of late-life depression linking it to cognitive impairment.
Depression and Cerebral Disruption
Depression is associated with disruption in a number of brain regions. Some described areas include the hippocampus, prefrontal systems and their limbic-hippocampal connections, the orbitofrontal cortex (OFC), the dorsolateral prefrontal cortex (DLPFC), the anterior cingulate gyrus, and the amygdala (
1,
59).
The hippocampus plays a critical role in learning, recall, and affect regulation (
1). Depression serves as a neurobiological stressor. Overall, it leads to excess circulating glucocorticoids, which result in hippocampal neurotoxicity and volume loss (
60). Decreased hippocampal volume may result from many factors. First, it has been demonstrated in animal studies that stress induces decreased cell proliferation in the hippocampal region, which leads to reduced neurogenesis (
61). Second, the glucocorticoid hypothesis (
62) postulates that a prolonged physical reaction to stress elicits sustained glucocorticoid secretion. There are glucocorticoid receptors in the hippocampus. Over time, glucocorticoid hypersecretion leads to excessive activation of these receptors. This overstimulation is eventually toxic and leads to neuronal cell death and atrophy. Such damage has been recorded in imaging studies (
63), has been associated with cognitive decline and dementia (
64,
65), and has been demonstrated in clinical studies (
5,
66).
Sheline et al. (
66) found that females, ages 23–86, with a history of depression (postdepression) had smaller hippocampal volumes bilaterally than did case-matched others without depression but with similar age and education. They scored lower on verbal memory, a neuropsychological measure of hippocampal function, which suggested that the volume loss was related to cognitive functioning. There was also a significant correlation with hippocampal volume loss and total lifetime duration of depression. This suggested a dose-response relationship between depression and cognitive impairment, which indicated that repeated stress from recurrent depressive episodes may result in cumulative hippocampal injury, reflected in volume loss (
66).
A later prospective study of elders found that over two years, the depressed group showed a greater reduction in left hippocampal volume than the nondepressed group. The study found that hippocampal change from baseline to two years was associated with subsequent change in Mini–Mental State Examination scores from two years to two and a half years in the depressed group. Thus, depressed elders with hippocampal volume loss were at greater risk of cognitive decline (
5). Age of depression onset seems to correlate negatively with hippocampal volume, so that patients with late-life depression have smaller hippocampal volumes compared with controls and those with early-onset depression (
59,
63). However, others have found different results and have connected reductions in hippocampal volume with earlier onset depression (
66) and longer duration of untreated depression (
67).
Last, with respect to other brain areas involving depression and cognition, the OFC seems to have a role in selectively processing affective stimuli. This makes depressed individuals predisposed to cognitive errors if they have received negative feedback on preceding tasks (
68). Increased lesion density has been found in the OFC among patients with late-life depression (
69). The anterior cingulate gyrus seems to be involved in initiation and suppression of behavior through conflict monitoring and appraisal of motivational content (
1). It is also involved in spatial planning and working memory. Individuals with depression have demonstrated lower activation of this region on the Tower of London task, which is a test of planning (
70). The DLPFC is involved in working memory, retrieval of episodic memory, planning, and response monitoring (
1). Late-life depression has also been associated with microstructural changes in the white matter of the DLPFC (
71).