Heightened adrenergic tone has been implicated in the pathophysiology of posttraumatic stress disorder (PTSD) (
1). Sympathetic nervous system abnormalities are thought to contribute to elevated daytime symptoms of PTSD, including the hyperarousal cluster of symptoms, as well as nighttime sleep disturbance, including nightmares and insomnia (
1–
4). Although the short-acting alpha-1 adrenergic receptor antagonist, prazosin (plasma half-life=two to three hours), has been demonstrated to be effective for PTSD nightmares in multiple small to medium-sized randomized controlled trials (
5–
7), a recent Veterans Affairs cooperative study of approximately 300 participants demonstrated no advantage of prazosin as compared with placebo, for either nighttime symptoms or PTSD symptoms overall (
8). Attention is now turning to an alternative adrenergic blocker, doxazosin, because of its unique pharmacokinetic properties, which may offer advantages over prazosin. Doxazosin has a long half-life of 15–22 hours and therefore potential benefit for daytime symptoms of PTSD (
9). Time to peak concentration is also two to three hours for doxazosin (vs. one hour for prazosin), which may enhance its effects on nightmares because nightmares are thought to occur during rapid eye movement sleep periods, which predominate later in the night.
Two pilot studies (including an open-label study and a small randomized trial involving a 16-day treatment period with a cross-over design) and one retrospective chart review indicate doxazosin may be effective for both PTSD and PTSD-related sleep disturbances (
10–
12). De Jong and colleagues (
10) performed a 12-week, open-label study of doxazosin extended-release (XL) for treating PTSD. In a sample of 12 participants, participants initiated doxazosin XL at 4 mg at bedtime daily, and increased to 8 mg at bedtime daily at week 8 in the absence of side effects (
10). The total Clinician-Administered PTSD Scale (CAPS [
13]) score declined from a mean of 77 (SD=14) at baseline to 55 (SD=22) at eight weeks and to 53 (SD=18) at 12 weeks. The combined CAPS sleep item scores (disturbed sleep and distressing dreams) decreased from a mean of 13.4 (SD=1.6) at baseline to 8.8 (SD=4.5) at the end of eight weeks of doxazosin treatment. They found that the medication was well-tolerated in their normotensive sample of patients with posttraumatic stress; they also found no significant changes in heart rate or blood pressure in response to the medication. Nonetheless, three patients experienced adverse effects at the higher dose of 8 mg daily and continued the study treatment at 4 mg daily. Two (
2) of the 12 participants stopped taking medication because of drowsiness, one after the first dose and one after ten weeks.
More recently, Rodgman and colleagues (
11) reported results from a double-blind, placebo-controlled, within-subject trial of doxazosin XL for PTSD that included eight male participants. In this study, participants were treated with doxazosin XL, titrated from 4 mg/day to 16 mg/day over 12 days, alternating with placebo, in a balanced cross-over design. Each treatment lasted 16 days and was separated by a two-week washout period. The authors did not find an effect of treatment, time, or treatment × time on CAPS scores; however, a treatment × time effect on hyperarousal scores, indicating an advantage of doxazosin over placebo, approached significance (p=.09). The authors also identified a treatment × time effect on PTSD Checklist (PCL) (
14) scores (p=.003), indicating an advantage of doxazosin over placebo. They reported no problems with tolerability of doxazosin at the target dose.
Finally, Roepke and colleagues (
12) reported findings from a retrospective chart review of doxazosin for nightmares among participants with PTSD or borderline personality disorder. In their analysis, 46 patients, predominantly female, started on doxazosin for prominent trauma-related nightmares in the context of PTSD and borderline personality disorder; they found that doxazosin immediate-release (IR) treatment was associated with a significant and clinically meaningful decrease in trauma-related nightmares, irrespective of diagnosis. Additionally, among 31 participants who completed 12 weeks of doxazosin treatment, 25% experienced a complete remission of trauma nightmares on the basis of the CAPS-IV distressing dreams assessment (
13). The average dose of doxazosin IR in that study was 5.28 mg at week 4 and 6.08 mg at week 12, with the maximum dose being 12 mg in one patient.
The reasons for discontinuation of doxazosin during treatment could not be clearly determined from the chart review; however, discontinuation due to “unspecific cardiac and circulatory disturbances” was identified as the reason for discontinuation for one patient. In that study, the IR version of doxazosin was used, which may have allowed for more fine-tuning of doxazosin dose in response to symptoms and side effects. The objectives of the current report are to present findings from an open-label pilot study of doxazosin XL for PTSD nightmares, sleep disturbance, and nonsleep PTSD symptoms and to discuss future directions for doxazosin research in light of existing findings.
Methods
Eligible participants were men and women age 18–69 with full or partial PTSD of at least three months’ duration and prominent nightmares, indicated by a CAPS-IV total score of ≥30 and a distressing dreams score of ≥5 (
13). Exclusion criteria included the following:
DSM-IV alcohol or drug abuse or dependence in the past 3 months; a lifetime history of schizophrenia or schizoaffective disorder; exposure to a criterion A trauma in the past three months; prominent suicidal or homicidal ideation; untreated sleep apnea or a positive screen for sleep apnea as determined by overnight assessment using a type III apnea screening device; a neurologic disorder or systemic illness known to affect central nervous system function; chronic or unstable medical illness, including unstable angina, myocardial infarction within the past six months, congestive heart failure, preexisting hypotension or orthostatic hypotension, chronic renal or hepatic failure, and pancreatitis; pregnancy, breastfeeding, or refusal to use effective birth control; previous serious adverse reaction to an alpha-1 antagonist; current use of trazodone, alpha-2 agonists, prazosin, or other alpha-1 antagonists; and previous nonresponse to prazosin for treatment of PTSD-related sleep disturbance. Participants on nonexclusionary psychiatric medications were permitted to participate as long as they were stable on their regimen for at least eight weeks and as long as they agreed to refrain from dose changes (absent any safety issues) for the duration of participation.
Written informed consent was obtained from all participants before eligibility assessments. All procedures were approved by, and performed in accordance with, the ethical standards of the ethical review board at the University of California, San Francisco. The trial consisted of baseline assessments (in week 0, which included seven days of sleep diary) followed by eight weeks of doxazosin treatment (weeks 1–8). Participants initiated doxazosin XL at the lowest available dose (4 mg) at bedtime, titrated up to 8 mg at bedtime in week 2 on the basis of clinical response and tolerability, and continued treatment on a steady dose for an additional six weeks for a total of eight weeks on the study drug. The primary endpoints were change in CAPS total, distressing dreams, and sleep disturbance scores from baseline (pretreatment) to end of treatment. Self-report measures of PTSD symptoms (PCL) (
14) and sleep quality (Pittsburgh Sleep Quality Index [PSQI]) (
15) were assessed at weeks 0 (pretreatment), 3, and 8. Sleep diary measures of number of nightmares and sleep quality on a Likert-type scale (0–100) were collected daily in weeks 0–8. Measures of depression (Beck Depression Inventory [BDI]) (
16) and quality of life (Quality of Life Inventory) (
17) were also assessed as secondary outcomes at weeks 0, 3, and 8.
Symptoms sometimes encountered as side effects to doxazosin, including dizziness when standing, lightheadedness, headache, drowsiness, lack of energy, weakness, palpitations, nausea, and swelling of the extremities, were assessed via clinical interview at all in-person visits as well as during midweek telephone visits during the two-week titration period (weeks 1–3). Participants were also asked whether any other symptoms, other than those listed, were present. Participants were asked whether the symptom was present or absent and, if present, whether it was acceptable (yes or no) as well as the start date and end date of the symptom. The study physician also made a determination regarding whether the symptom was drug related (yes or no) on the basis of whether the symptom was present before study drug initiation or whether it seemed worse or substantively different than before study medication initiation. The physician recorded whether the medication was or was not thought to be related to the study drug.
Given the potential effects of alpha-1 blockade on blood pressure, including orthostatic hypotension, blood pressure and heartrate were also assessed at all in-person visits. Participants were first assessed in a reclined position and then in the standing position after two minutes of standing up.
Statistical Analysis
Because we cannot assume normality of error distributions, we used nonparametric statistics for analyses of continuous outcome variables. Wilcoxon signed-rank tests were used to compare week 0 versus week 8 scores on CAPS-derived variables (total score, distressing dreams, and sleep disturbance). The PCL (PTSD), PSQI (sleep quality), BDI (depression), and Quality of Life Inventory (quality of life) scores were analyzed using the Skillings-Mack (SM) test, which is an extension of the Friedman test that can accommodate missing data and unequal cell sizes (with complete balanced data the two tests are identical) (
18,
19). From the sleep diary, the total number of nightmares per week and the mean sleep quality rating per week were calculated for each participant. SM tests were used to assess changes in diary sleep quality ratings over time, and a mixed-effects Poisson regression model, appropriate for count data, was used to analyze change in number of nightmares over time. The Poisson mixed model treated week as a categorical fixed variable to allow for nonlinear changes over time.
All participants who contributed any data beyond baseline were included in the analyses despite missing data at some time points. For the SM tests, p values were based on simulations under the null distribution, as implemented in the Stata, version 14, “skilmack” command. Simulations are recommended to provide more accurate p values than the chi-square approximation when sample size is not large (
19). Similarly, nonparametric bootstrap resampling was used to estimate confidence intervals presented in graphs.
Results
Fifteen participants initiated study drug, of whom eight completed all eight weeks of the study drug and provided CAPS data for primary outcome analysis. The completer sample consisted of eight adults (63% female), with a mean age of 40.1 years (SD=14.8). Nine out of 15 participants enrolled had full syndromal PTSD, whereas the rest satisfied inclusion criteria on the basis of nightmare severity and a CAPS score of at least 30 (
Table 1). An additional male participant completed two of three visits and provided subjective report survey data at week 3 (after two weeks on the study drug). Additionally, diary data beyond baseline were gathered on 11 out of 15 enrollees. There were no significant age or sex differences between completers and noncompleters.
Several participants were taking nonexclusionary medications, including one completer taking 15 medications for a mix of mental-health and non–mental health conditions. The following psychotropic medications were taken by participants: buspirone (one participant), aripiprazole (one participant), duloxetine (one participant), mirtazapine (one participant), escitalopram (one participant), and melatonin (one participant). One participant missed a single dose of mirtazapine after stopping because of perceived lack of indication while on doxazosin. The participant reinitiated mirtazapine to qualify for continuation in the study. No other medication changes (of either psychotropic or other medications) were reported by participants in the study.
Among the seven participants who discontinued before completion of treatment, four participants (three women, one man) discontinued because of subjective side effects. Discontinuation due to side effects occurred shortly after medication initiation, ranging between one dose (N=1), two doses (N=1), six doses (N=1), and nine doses (N=1) of the study drug. These four individuals reported the following symptoms, queried using a survey on the most common side effects of doxazosin, at the final contact with study staff: drowsiness (N=2), low energy (N=3), weakness (N=2), nausea (N=1), nasal congestion (N=1), headache (N=3), subjective palpitations (N=1), and increased heart rate (N=1). Symptoms including drowsiness and low energy frequently preceded doxazosin initiation and could not be attributed confidently to the study drug. Three additional participants stopped for reasons unrelated to the study drug, including concern about potential side effects after reading the information packet following a single dose, in the absence of side effects (N=1); inability to continue because of travel (N=1); and loss to follow-up in a participant known to the investigators as having a history of poor mental health treatment follow-up.
Among completers, average pretreatment CAPS total, distressing dreams, and sleep disturbance scores decreased from 57.3 (SD=8.1) to 31.5 (SD=14.5) (z=2.52, p=.012); 5.5 (SD=.78) to 2.4 (SD=2.6) (z=2.49, p=.013); and 6.6 (SD=.91) to 4.3 (SD=3.2) (z=1.60, p=.110), respectively. SM tests including all nine participants who provided data beyond baseline demonstrated significant improvement on the PCL (average drop of 18.7 points; SM=10.88, p=.002) and PSQI (average drop of 4.1 points; SM=2.00, p=.030). Sleep diary data including all 11 participants providing diary data beyond baseline demonstrated a significant reduction in nightmares (z=−2.8, p=.006) and improvement in sleep quality (SM=3.1, p=.014) from baseline to end of treatment. Post hoc analyses examining the relationship between doxazosin treatment and reductions in intrusion, avoidance, and hyperarousal clusters of PTSD symptoms separately indicated that declines in all three symptom clusters were statistically significant.
For secondary outcomes of depression and quality of life, doxazosin XL was also associated with improvements. For the BDI (depression) scores, a clinically and statistically significant decrease of 13.9 points on average (SM=9.27, p=.010) was observed. Quality of life scores increased an average of 26.2 points, a clinically relevant but non–statistically significant effect (SM=3.70, p=.161).
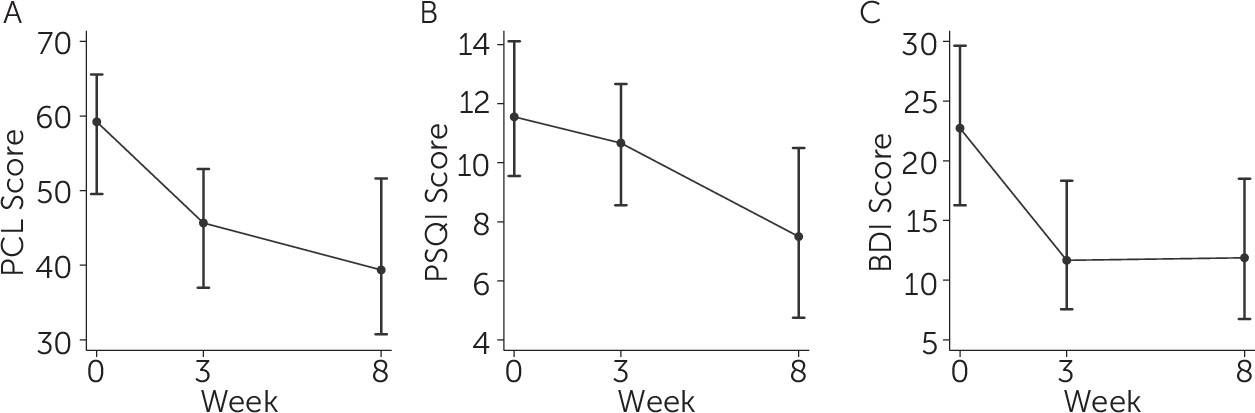
Examination of the timeframe for symptom change using the outcomes for which more than two timepoints were available (PCL, PSQI, BDI, and sleep diary) indicate that most benefit occurred by the end of the second week of treatment, after which point treatment gains were either sustained or mildly enhanced. See
Figure 1 for depictions of the time course of change for PTSD symptoms (PCL score;
Figure 1A), subjective sleep quality (PSQI score;
Figure 1B), and depression symptoms (BDI score;
Figure 1C). As depicted in
Figure 1B, the difference between week 3 and week 8 for PSQI sleep quality was significant (SM=2.0, p=.030).
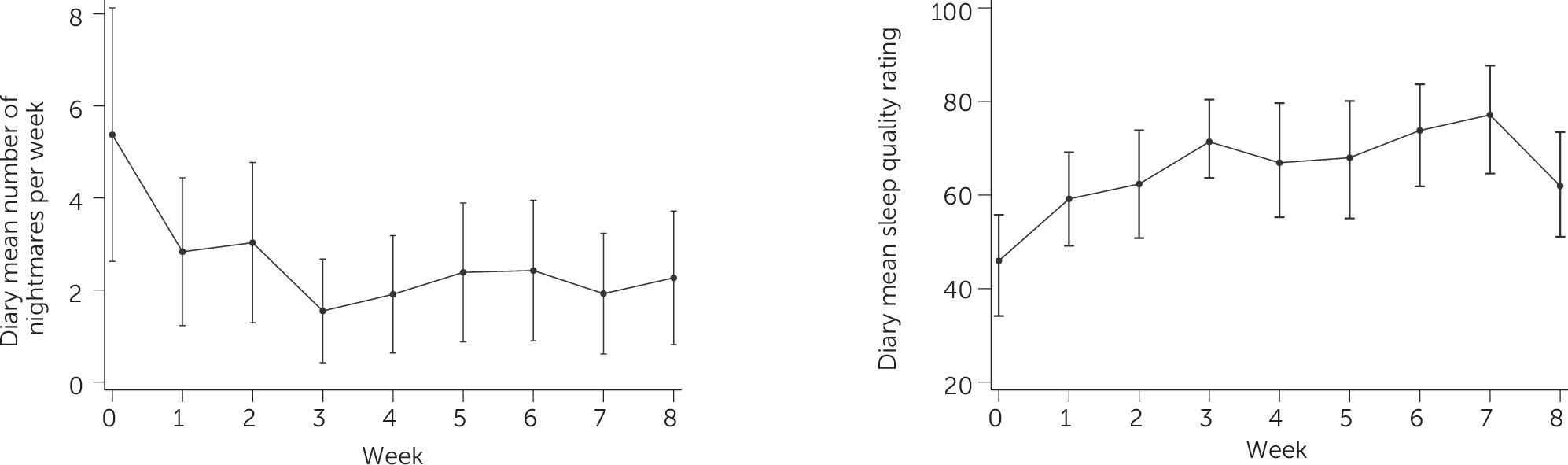
Diary-based measures of nightmare number and sleep quality are depicted in
Figure 2. There was a dip in mean sleep quality in week 8, the reason for which is unclear. This finding conflicts with the PSQI score changes, which demonstrated ongoing improvements over the course of treatment. The improvement in mean sleep quality between baseline and week 8 was nonetheless statistically significant.
Post hoc analyses examined whether baseline blood pressure and heart rate predicted drop out or treatment response. Baseline blood pressure and heart rate were calculated as the average of two measures, collected at the eligibility visit and at the first medication visit prior to drug initiation. Although there were no differences in baseline sitting or standing diastolic or systolic blood pressure between those who discontinued because of side effects and other participants, there was a statistically significant difference between baseline standing heart rate in those who ended up discontinuing because of side effects (mean heartrate=88 bpm) as compared with others (mean heartrate=75 bpm; z=2.56, p=.010).
Furthermore, there was a statistically significant difference in baseline orthostatic systolic blood pressure changes, with those discontinuing because of side effects displaying a 5.3-mmHg average decrease in systolic blood pressure between supine and standing positions and all others displaying a 0.45-mmHg average increase in systolic blood pressure between supine and standing positions (z=2.85, p=.004). None of the participants discontinuing because of side effects met criteria for orthostatic hypotension, defined as a drop of systolic blood pressure of ≥20 mmHg or diastolic blood pressure of ≥10 mmHg from supine to standing, or orthostatic heart rate changes, defined as an increase in heart rate of >20 bpm between supine and standing positions at the time of their last completed study visit; however, only one of four participants completed an in-person discontinuation visit. We also did not find that baseline blood pressure or heart rate variables predicted treatment response.
Discussion
This study adds to the small body of research pointing to doxazosin as a potential treatment for PTSD. The sizeable improvements in nightmares and nonnightmare sleep disturbance, as well as overall PTSD symptoms, are consistent with prior studies of prazosin and the open-label study of doxazosin conducted by De Jong and colleagues (
5–
7,
10–
12). However, the CAPS effect we observed was greater than that reported by Rodgman and colleagues (
11). De Jong et al.’s (
10) protocol followed participants for 12 weeks of treatment and assessed CAPS PTSD symptoms at eight and 12 weeks of treatment, whereas Rodgman et al. followed participants for only 16 days and performed CAPS assessments at four time points (at baseline and end of treatment for each treatment, doxazosin and placebo, in the cross-over design). Because we did not perform interim CAPS assessments, it is difficult to compare results between our study with those of Rodgman et al.’s study. However, comparison between week 3 versus baseline changes in PCL score in our study and end of treatment effects in Rodgman et al.’s 16-day study appear consistent with each other. Although Rodgman et al. could not fully explain the discrepancy between the CAPS and PCL results in their study, some researchers have suggested that the PCL score may be more sensitive than the CAPS to treatment differences in PTSD (
11,
20).
Interestingly, in the current study, the benefits of doxazosin manifested, for the most part, within the first two weeks of treatment. Although participants sustained improvement, differences in symptom levels between week 8 and week 3 were only statistically significant for the PSQI sleep quality score. These findings contrast with findings reported by Rodgman et al. (
11), who found only small, statistically nonsignificant improvements in CAPS PTSD symptoms after a 16-day treatment with doxazosin, on the basis of which they recommended longer term treatment. Of note, their study only included male participants, whereas most completers in the current study were female.
This study benefited from weekly sleep diary, which provided a more reliable, day-to-day assessment of symptom change. Sleep diary analysis included both completers and noncompleters and indicates that noncompleters experienced some improvement in their nightmares and sleep quality while on the study medication. The current study benefited from different approaches for gathering nightmare, sleep quality, and PTSD symptom data. Analysis of secondary self-report measures provided internal validation of our primary findings and strengthened our confidence in our findings. Future research on sleep in PTSD should take advantage of modern technologies, such as app-based sleep diaries, which can provide time-stamped, daily, subjective report data to further enhance validity of sleep-wake activity data.
The improvement in depression score was statistically significant and clinically meaningful. Studies to date have either not reported on (
10,
12), or have not found (
11), improvements in depression. Although it is possible that doxazosin has a direct effect on depression symptoms, there is no known biological mechanism with which to explain this. It is more likely that effects on depression were mediated by the effects of doxazosin on PTSD symptoms. Future studies should examine the effects of doxazosin on depression because depression is so often comorbid with PTSD, and it will be important to examine whether effects on depression are replicated and whether they can be explained by effects on PTSD symptoms.
Although improvements on doxazosin in study completers were reliably found in multiple symptom domains, and although sleep diary data indicate that even noncompleters may have experienced PTSD and sleep symptom reduction on the study medication, four out of 15 participants (27%) discontinued because of side effects. The current study used doxazosin XL, for which only 4- and 8-mg formulations are available. Future research should examine the IR version of doxazosin. Although it requires a slower, more gradual titration similar to prazosin, initiation at 1 mg allows for a lower starting dose and more gradual and flexible increase. Although Rodgman et al. (
11) reported a successful titration of doxazosin to 16 mg over 12 days, this may not be feasible across the board. Because the IR and XL formulations of doxazosin have similar half-lives, the only clear advantage of the XL formulation is the ease of initiation (at a higher, potentially therapeutic dose). The limited range of doses, however, may be a significant barrier to tolerability.
Of note, three out of four noncompleters who discontinued because of side effects in the current study were female. Although our sample is too small to examine sex differences, it is important to note that Rodgman et al.’s (
11) study included only male participants. The average doxazosin IR dose for participants in the retrospective chart review by Roepke and colleagues (
12), who studied a patient sample that was 92% female, was 5.3 mg at week 4 and 6.1 mg at week 12. It is becoming more evident that the safety window for men and women may differ for some medications; therefore, future studies should examine gender differences in tolerability and response to doxazosin. Use of the IR formulation will allow for the flexibility of starting with a low dose and making fine adjustments upward to reduce side effects. An additional major advantage of the IR formulation, with respect to access to care, is its low cost and availability in most nonspecialty settings.
Additionally, two mental health treatment studies have indicated that elevated pretreatment blood pressure predicts response to treatment by alpha-1 blockers. One study found that pretreatment standing systolic blood pressure predicted response to prazosin for PTSD (
21). Another study found that pretreatment standing diastolic blood pressure predicted response to doxazosin for the treatment of alcohol use disorder (
22). We did not find an association between pretreatment blood pressure and symptom change on doxazosin. It is likely that such a relationship was difficult to detect in such a small sample. Future research with larger samples and greater variability in blood pressure will allow for further examination of this topic.
A limitation of the study was that we did not include a quantifiable measure of side-effect severity, rendering it difficult to determine whether drowsiness, lack of energy, and fatigue, which may manifest as a consequence of PTSD, were rendered worse by doxazosin treatment because side effects of doxazosin can also include these same symptoms. Future studies of doxazosin should include quantifiable measures, such as the Epworth Sleepiness Scale (
23), to assess these symptoms on a regular basis using a more fine-grained and quantifiable approach to determine relationship to the study drug.
Although findings in the current study are intriguing, and enhance the rationale for further study of doxazosin for treating PTSD, it is important to emphasize the limitations of open-label studies such as this one. Because both patients and prescribers are aware of what medication the patient is taking, one cannot claim that the research team’s interest in studying a potentially new treatment has no influence of patient reports of outcome. Nonetheless, the clinical interviewers performing CAPS assessments work on diverse studies, have limited awareness regarding the goals of the study, and have no specific interest in outcomes, which enhances our confidence in the validity of observed CAPS symptom changes. Additionally, patients in the study had regular contact with study staff, which may in and of itself have produced a therapeutic effect. Future studies should include randomized, double-blind, controlled designs in which clinical assessments are performed by interviewers who are blinded, as much as possible, to the nature and objectives of the study.
Conclusions
These findings in a small sample of participants with PTSD demonstrate promising symptom improvement during eight weeks of doxazosin treatment. Benefits were also seen in noncompleters. Although several studies have demonstrated tolerability of doxazosin, these data indicate that doxazosin may be beneficial for PTSD symptoms but that tolerability for the XL formulation may be a problem. Baseline blood pressure and heartrate variables may predict tolerability. Further research that includes adequately powered, randomized, placebo-controlled studies in which both men and women participate should be performed, and the IR formulation that allows for more gradual and flexible titration should be studied. In step with the precision-medicine objectives of individualizing patient care, future research should include examination of moderators of doxazosin treatment tolerability and treatment effects.